In recent decades, the contamination by heavy metals has become a global issue of great concern because of their serious threat to ecosystem and human health [1]. Silver (Ag) is always considered to be a precious metal harmless to human and it has been frequently used in our daily life, such as decoration and agent to treat diseases. It also has widespread applications in many industrial fields including catalysis, electronics, photography, imaging, medical science, and so on. Recently, however, the more and more attention has been paid to the negative effect of Ag. Ag ion (Ag+) has been regarded as one of the most hazardous heavy metal pollutants, which can cause many adverse effects on the environment [2]. In addition, Ag+ can bind to various metabolites such as imidazole, thiol, amino, and carboxyl groups in proteins/enzymes, thereby seriously disturbing protein/enzyme function and causing many symptoms and diseases [3]. Accordingly, the design and development of a simple technique capable of selective Ag+ determination with high sensitivity is of paramount significance for environmental monitoring and public health.
Numerous techniques, mainly including atomic absorption/emission spectroscopy [4], inductively coupled plasma mass spectrometry [5], inductively coupled plasma optical emission spectrometry [6], electrochemical methods [7, 8], surface-enhanced Raman spectroscopy [9], and fluorescence spectroscopy [10] have been employed to the determination of Ag+. Nevertheless, the range of their practical applications is severely limited by the requirements of expensive and non-portable instrumentations, elaborate sample preparation, time-consuming analysis, and skilled operators. In response to these shortcomings, colorimetric methods for Ag+ detection have been developed. This approach is extremely convenient, easy to be performed by inexpensive spectrophotometer or naked eyes, and do not need of professional operation. To date, plasmonic gold nanoparticles (GNPs) are the most commonly used optical nanomaterials for colorimetric sensing of Ag+ [11-15]. The GNPs-based colorimetric assays typically rely on distinct color changes of GNPs aggregation (or redispersion of GNPs aggregate) owing to their high extinction coefficients and strong surface plasmon resonance (SPR) absorptions in the visible region. However, in such assays, a complex modification of GNPs is usually necessary to obtain high selectivity towards Ag+, which significantly increases time and cost and thus restricts their applicability to some extent. Furthermore, most of current plasmonic GNPs-based colorimetric detection methods lack sufficient sensitivity. As a result, it is still a great challenge to develop simple but efficient method that can achieve sensitive and selective detection of Ag+.
Over the past few years, nanomaterial-based artificial enzymes, the so-called nanozymes, have evoked much research interest due to their facile synthesis, easy modification, excellent operational stability, low cost, and tunable catalytic activity which are beneficial to the real application [16-19]. Until now, a variety of nanozymes have been reported to successfully mimic different natural enzymes, such as peroxidase, catalase, superoxide dismutase, oxidase, nitrate reductase, phosphatase, urease, protease and nuclease [20]. Unlike peroxidase, which requires hydrogen peroxide (H2O2) as an electron acceptor, oxidase can initiate the catalytic oxidation of organic substrates employing molecular oxygen (O2) as a green oxidant. Consequently, a nanozyme with oxidase-mimicking activity provides a promising alternative for designing and constructing methods with convenient operation, good reliability, and outstanding compatibility. Oxidase mimicking CeO2 nanoparticles, MnO2 nanoparticles, MnCo2O4 nanofibers, CoOOH nanoflakes, gold nanoclusters, and Au@Pt nanostructures have been utilized for the detection of cancer biomarkers, alkaline phosphatase, DNA, melamine, biothiols, ions, and so on [21-26].
In our previous work, we have demonstrated a simple approach for the preparation of platinum nanoparticles (PtNPs) using chitosan (Ch) as a stabilizing agent [27]. The resulting Ch-PtNPs have been found to possess intrinsic oxidase-like activity, which can catalyze the fast oxidation of the substrate 3, 3', 5, 5'-tetra-methylbenzidine (TMB) by dissolved O2 and lead to the formation of a colored product. Compared to other kinds of oxidase mimetics, Ch-PtNPs exhibit a higher binding affinity for TMB. In this contribution, we observed that Ag+ can effectively inhibit the TMB oxidation reaction catalyzed by Ch-PtNPs.
As seen in the inset of Fig. 1, an intense blue color was found in Ch-PtNP/TMB system, suggesting that Ch-PtNPs possess high oxidase-mimicking activity. Surprisingly, after introduction of 1000 nmol/L Ag+, the catalytic activity of Ch-PtNPs decreased distinctly, and nearly no colored derivative was produced. We recorded the UV–vis absorption spectra of these samples. As displayed in Fig. 1, oxidized TMB (oxTMB) shows an obvious peak at around 652 nm, whose height is approximately 52 times lower with Ag+ than without it. The inhibition effect of Ag+ is fairly universal for different types of substrates. After the addition of Ag+, the oxidation of other chromogenic reagents, including 2, 2'-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and o-phenylenediamine (OPD), catalyzed by Ch-PtNPs was also significantly suppressed (Fig. S1 in Supporting information). The Ch-PtNPs have a high ratio of Pt2+ (4f145d8) species on the NP surface (45%) [27], which can combine with Ag+ (4d10) through the d8-d10 metal-metal interaction [28]. The metallophilic bonding of d8-d10 exhibits the similar characteristic to the d8-d8 and d10-d10 cases, and its strength is stronger than van der Waals force and similar to the medium hydrogen bond [28]. The adsorption of Ag+ on the metal core of Ch-PtNPs can weaken the affinity to the substrates and eventually inhibit their oxidasemimicking activity [29, 30].

|
Download:
|
| Fig. 1. UV–vis absorption spectra of (a) Ch-PtNPs +0.15 mmol/L TMB and (b) ChPtNPs +0.15 mmol/L TMB + 1000 nmol/L Ag+. Inset: the corresponding photographs. Experiments were performed in 20 mmol/L phosphate buffer (pH 4.5) at 50 ℃ for 20 min. | |
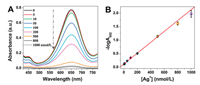
As described above, the catalytic activity of oxidase-mimicking Ch-PtNPs can be inactivated by Ag+, and its inhibition efficiency is directly dependent on the concentration of Ag+. The more Ag+ ions exist in the testing solution, the smaller absorbance signal is generated from the Ch-PtNP/TMB oxidation reaction. Therefore, we can use the Ch-PtNP/TMB system for colorimetric detection of Ag+. For fast and sensitive detection of Ag+, the incubation time was selected as 20 min (Fig. S2 in Supporting information). First, the sensitivity of the detection system was investigated by assaying solutions with various Ag+ concentrations (0, 5, 10, 50, 100, 200, 500, 800, and 1000 nmol/L). As depicted in Fig. 2A, with the increasing concentration of Ag+, the absorbance at 652 nm (A652) of Ch-PtNPs-catalyzed TMB reporting system gradually declined. A good linear relationship can be found between the negative logarithm of A652 (-logA652) and Ag+ concentration in the range of 5–1000 nmol/L (-logA652 = 0.00201[Ag+] + 0.11684) with a correlation coefficient (r) of 0.9942 (Fig. 2B). The limit of detection (LOD) of this approach was calculated to be 4 nmol/L by using the equation LOD = 3S/K, where S represents the standard deviation of the blank signal (0.00272) and K is the slope of the working curve (0.00201). This value was far below the maximum allowable level of Ag+ ions (0.05 mg/L = 460 nmol/L) in drinking water set up by the United States Environmental Protection Agency (EPA) [31]. The relative standard deviation (RSD) for nine repeated measurements of 100 nmol/L of Ag+ was found to be 1.9%, which indicated that the response of Ch-PtNP/TMB system towards Ag+ was highly reproducible.
|
Download:
|
| Fig. 2. (A) UV–vis absorption spectra of the Ch-PtNP/TMB reporting system after the addition of different concentrations of Ag+. (B) The linear calibration plot for Ag+ detection. The vertical bars represent the standard deviation for three independent replications. Experiments were performed in 20 mmol/L phosphate buffer (pH 4.5) at 50 ℃ for 20 min. | |
In order to spotlight the remarkable advantages of this colorimetric assay, its analytical performance was compared with those of previously reported optical methods for the determination of Ag+. As can be seen from Table 1 [12-14, 32-37], the LOD of our method was comparable to or even lower than most fluorescent and colorimetric methods documented in the literature. Such a high detection sensitivity of our sensing system can be attributed to the excellent oxidase-mimicking activity of Ch-PtNPs and the powerful inhibition effect of Ag+ on Ch-PtNPs-catalyzed oxidation reaction of TMB, which together resulting in an amplified signal diminishment process. In addition, the proposed method does not involve any carefully controlled modifying or labeling procedures of nanoprobes for specific recognition, which dramatically simplified the analysis process. Besides, because of the amplification effect of the catalytic reaction, only tiny amounts of Pt are needed in the assay, thus enabling a cost-effective way for Ag+ monitoring. Furthermore, our method is more reliable and compatible since the catalytic oxidation of TMB can be triggered without the assistance of strong oxidant H2O2 which can be readily decomposed.
|
|
Table 1 Comparison of optical methods for the detection of Ag+ ions. |
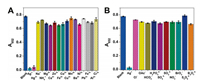
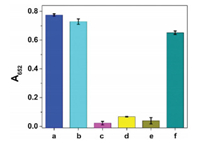
Next, the anti-interference capability of this sensing system was evaluated. Common cations (Hg2+, Na+, NH4+, Mg2+, Ca2+, Zn2+, Fe2+, Co2+, Cu2+, Mn2+, Ba2+, Ni2+, Pb2+, Fe3+, Al3+, and Cr3+) and anions (Cl-, OAc-, HCO3-, H2PO4-, SO42-, SO32-, NO3-, BrO3-, S2O82-, and B4O72-) as interferents were examined. From Fig. 3, we can see that a significant decrease in absorbance signal is observed for Ag+, whereas most of other ions under the same concentration show negligible effect on A652 value. Note that Hg2+ also can cause a decrease of absorbance (Fig. 3A), which is probably due to the fact that the ionic radius of Hg2+ is close to that of Ag+ (0.110 nm vs. 0.126 nm) and it also has d10 electron configuration [38, 39]. However, the addition of EDTA can perfectly mask the interference from Hg2+ while has little influence on the Ag+-triggered inhibition of the color reaction (Fig. 4). This is because the conditional stability constant of EDTA-Hg2+ is more than 1010-fold higher than that of EDTA-Ag+ [40]. These results sufficiently illustrated that the oxidase-mimicking Ch-PtNPs based colorimetric method is highly selective for Ag+ detection and can be capable of resisting interference.
|
Download:
|
| Fig. 3. Selectivity test of the proposed sensing system for the detection of Ag+. (A) Cations and (B) anions. The concentration of all the evaluated ions was 1000 nmol/L. The vertical bars represent the standard deviation for three independent replications. Experiments were performed in 20 mmol/L phosphate buffer (pH 4.5) at 50 ℃ for 20 min. | |
|
Download:
|
| Fig. 4. Influences of Ag+ and Hg2+ on the Ch-PtNP/TMB reporting system in the presence of EDTA (100 μmol/L). (a–f): Ch-PtNPs + TMB, Ch-PtNPs + TMB + EDTA, ChPtNPs + TMB + Ag+, Ch-PtNPs + TMB + Ag+ + EDTA, Ch-PtNPs + TMB + Hg2+, ChPtNPs + TMB + Hg2+ + EDTA. The concentrations of Ag+ and Hg2+ are both 1000 nmol/L. The vertical bars represent the standard deviation for three independent replications. | |
To further confirm whether the proposed colorimetric method had potential practical application, Ag+ detection in stimulated polluted samples (by adding Ag+ in tap and lake waters) was performed. Three different concentrations of Ag+ were respectively spiked into water samples and then analyzed by using the standard addition method. As shown in Table S1 (Supporting information), the recoveries for all the samples were in the range of 97.1%- 109.4% with RSDs from 0.8% to 8.5%, which testifying that no significant differences were found between the measured and added values. This result demonstrates that the presented sensing strategy is feasible for Ag+ detection in real-life sample.
In summary, by taking advantage of the oxidase-like activity of the Ch-PtNPs, a facile colorimetric method for the detection of Ag+ is successfully developed. The Ag+ ions can selectively bind to the surface of Ch-PtNPs through metallophilic Pt2+-Ag+ interactions and block the active sites for catalysis, thus leading to an apparent restriction of the catalytic activity of the Ch-PtNPs. The linear range for Ag+ determination is 5–1000 nmol/L and the LOD is 4 nmol/L. The high specific metallophilic interaction offers good selectivity over other tested ions. Moreover, the proposed sensing system has been applied to monitor Ag+ concentration in tap and lake waters with satisfactory results. In view of the high sensitivity, excellent selectivity, cost-effectiveness, rapidness, and simplicity, we believe that this colorimetric assay is expected to have great potentials in environmental monitoring and medical diagnosis.
AcknowledgmentsThe authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21075023, 21804021), the Program for Innovative Leading Talents in Fujian Province (No. 2016B016), the Joint Funds for the Innovation of Science and Technology, Fujian Province (No. 2016Y9056), the Natural Science Foundation of Fujian Province (No. 2017J01575), and Startup Fund for Scientific Research, Fujian Medical University (No. 2017XQ1014).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.05.032.
| [1] |
M. Li, H.L. Gou, I. Al-Ogaidi, N.Q. Wu, ACS Sustain. Chem. Eng. 1 (2013) 713-723. DOI:10.1021/sc400019a |
| [2] |
M.J. Eckelman, T.E. Graedel, Environ. Sci. Technol. 41 (2007) 6283-6289. DOI:10.1021/es062970d |
| [3] |
P.L. Drake, K.J. Hazelwood, Ann. Occup. Hyg. 49 (2005) 575-585. |
| [4] |
G. Chakrapani, P.L. Mahanta, D.S.R. Murty, B. Gomathy, Talanta 53 (2001) 1139-1147. DOI:10.1016/S0039-9140(00)00601-9 |
| [5] |
F. Laborda, J. Jimenez-Lamana, E. Bolea, J.R. Castillo, J. Anal. Atom. Spectrom. 26 (2011) 1362-1371. DOI:10.1039/c0ja00098a |
| [6] |
S.W. Brittle, J.D. Baker, K.M. Dorney, et al., J. Chem. Edu. 92 (2015) 1061-1065. DOI:10.1021/ed500707k |
| [7] |
G.P. Yan, Y.H. Wang, X.X. He, et al., Talanta 94 (2012) 178-183. DOI:10.1016/j.talanta.2012.03.014 |
| [8] |
A.L. Sun, F.C. Jia, Y.F. Zhang, X.N. Wang, Analyst 140 (2015) 2634-2637. DOI:10.1039/C5AN00046G |
| [9] |
E.Z. Tan, P.G. Yin, X.F. Lang, et al., Analyst 137 (2012) 3925-3928. DOI:10.1039/c2an35670h |
| [10] |
H. hang, L.X. Xie, W.B. Yan, et al., New. J. Chem. 33 (2009) 1478-1481. DOI:10.1039/b904369a |
| [11] |
D. Le, H. Lee, G. Lee, et al., Nanotechnology 30 (2018) 085501. |
| [12] |
X.Y. Li, Z.T. Wu, X.D. Zhou, J.M. Hu, Biosens. Bioelectron. 92 (2017) 496-501. DOI:10.1016/j.bios.2016.10.075 |
| [13] |
C.Y. Lin, C.J. Yu, Y.H. Lin, W.L. Tseng, Anal. Chem. 82 (2010) 6830-6837. DOI:10.1021/ac1007909 |
| [14] |
A. Safavi, R. Ahmadi, Z. Mohammadpour, Sens. Actuat. B-Chem. 242 (2017) 609-615. DOI:10.1016/j.snb.2016.11.043 |
| [15] |
M.L. Zhou, T. Lin, X.X. Gan, Microchim. Acta 184 (2017) 4671-4677. DOI:10.1007/s00604-017-2518-3 |
| [16] |
J.J.X. Wu, X.Y. Wang, Q. Wang, et al., Chem. Soc. Rev. 48 (2019) 1004-1076. DOI:10.1039/C8CS00457A |
| [17] |
J. Chen, Q. Chen, J.Y. Chen, H.D. Qiu, Microchim. Acta 183 (2016) 3191-3199. DOI:10.1007/s00604-016-1972-7 |
| [18] |
Q. Chen, J. Chen, C.J. Gao, et al., Analyst 140 (2015) 2857-2863. DOI:10.1039/C5AN00031A |
| [19] |
C.J. Gao, H.M. Zhu, J. Chen, H.D. Qiu, Chin. Chem. Lett. 28 (2017) 1006-1012. DOI:10.1016/j.cclet.2017.02.011 |
| [20] |
Y. Huang, J. Ren, X. Qu, Chem. Rev. 119 (2019) 4357-4412. DOI:10.1021/acs.chemrev.8b00672 |
| [21] |
A. Asati, C. Kaittanis, S. Santra, J.M. Perez, Anal. Chem. 83 (2011) 2547-2553. DOI:10.1021/ac102826k |
| [22] |
X. Liu, Q. Wang, H.H. Zhao, et al., Analyst 137 (2012) 4552-4558. DOI:10.1039/c2an35700c |
| [23] |
M. Gao, X.F. Lu, M.Q. Chi, S.H. Chen, C. Wang, Inorg. Chem. Front. 4 (2017) 1862-1869. DOI:10.1039/C7QI00458C |
| [24] |
S.G. Liu, L. Han, N. Li, et al., J. Mater. Chem. B 6 (2018) 2843-2850. DOI:10.1039/C7TB03275G |
| [25] |
G.X. Cao, X.M. Wu, Y.M. Dong, Z.J. Li, G.L. Wang, Microchim. Acta 183 (2016) 441-448. DOI:10.1007/s00604-015-1669-3 |
| [26] |
W.W. He, Y. Liu, J.S. Yuan, et al., Biomaterials 32 (2011) 1139-1147. DOI:10.1016/j.biomaterials.2010.09.040 |
| [27] |
H.H. Deng, X.L. Lin, Y.H. Liu, et al., Nanoscale 9 (2017) 10292-10300. DOI:10.1039/C7NR03399K |
| [28] |
B.H. Xia, H.X. Zhang, Y.Q. Jiao, et al., J. Chem. Phy. 120 (2004) 11487-11492. DOI:10.1063/1.1753563 |
| [29] |
X.M. Shen, W.Q. Liu, X.J. Gao, et al., J. Am. Chem. Soc. 137 (2015) 15882-15891. DOI:10.1021/jacs.5b10346 |
| [30] |
G.W. Wu, S.B. He, H.P. Peng, et al., Anal. Chem. 86 (2014) 10955-10960. DOI:10.1021/ac503544w |
| [31] |
Z. Xu, L.G. Xu, L.M. Liz-Marzan, et al., Adv. Optical Mater. 1 (2013) 626-630. DOI:10.1002/adom.201300148 |
| [32] |
X.E. Zhao, C.H. Lei, Y. Gao, et al., Sens. Actuat. B-Chem. 253 (2017) 239-246. DOI:10.1016/j.snb.2017.06.086 |
| [33] |
X.H. Gao, Y.Z. Lu, R.Z. Zhang, et al., J. Mater. Chem. C 3 (2015) 2302-2309. DOI:10.1039/C4TC02582B |
| [34] |
J. Lee, J. Park, H.H. Lee, et al., Biosens. Bioelectron. 68 (2015) 642-647. DOI:10.1016/j.bios.2015.01.058 |
| [35] |
J.H. Wang, H.Q. Wang, H.L. Zhang, et al., Anal. Bioanal. Chem. 388 (2007) 969-974. DOI:10.1007/s00216-007-1277-0 |
| [36] |
Y. Yue, T.Y. Liu, H.W. Li, Z.Y. Liu, Y.Q. Wu, Nanoscale 4 (2012) 2251-2254. DOI:10.1039/c2nr12056a |
| [37] |
Y.Q. Chang, Z. Zhang, J.H. Hao, W.S. Yang, J.L. Tang, Sens. Actuat. B-Chem. 232 (2016) 692-697. DOI:10.1016/j.snb.2016.04.039 |
| [38] |
N.T.K. Dung, R. Ludwig, New J. Chem. 23 (1999) 603-607. DOI:10.1039/a809771b |
| [39] |
S. Huang, Z. Wen, X. Zhu, Z. Gu, Electrochem. Commun. 6 (2004) 1093-1097. DOI:10.1016/j.elecom.2004.08.013 |
| [40] |
B.L. Li, Y. Du, S.J. Dong, Anal. Chim. Acta 644 (2009) 78-82. DOI:10.1016/j.aca.2009.04.022 |
 2019, Vol. 30
2019, Vol. 30 


