b Department of Applied Chemistry, School of Science, Xi'an Jiaotong University, Xi'an 710049, China
Serotonin (5-hydroxytryptamine, 5-HT), one of the monoamine neurotransmitters, plays an important role in the regulation of numerous behavioral and physiological functions such as human mood, alertness, appetite and sleep [1]. Low level of 5-HT in the body is associated with several diseases and disorders, such as Alzheimer's disease, sleep disorders, depression, unregulated hemostasis, and sudden infant death syndrome, while high level of serotonin in the body may lead to carcinoid syndrome [2]. Therefore, it is necessary to develop simple, selective and sensitive methods for the detection of 5-HT in biological systems.
A variety of methods for the detection of 5-HT were developed, such as capillary electrophoresis [3], enzyme immunoassay [4], high performance liquid chromatography [5], liquid chromatograph-mass spectrometer [6], fluorescence [7], chemiluminescence [8] and electrochemical method [9]. Among them, electrochemical methods received much attention in the detection of 5-HT due to their high sensitivity, low cost, simplicity and fast response. However, there are several challenges in the electrochemical detection of 5-HT. One is the interferences from other electroactive biomolecules in biological samples such as ascorbic acid (AA), 5-hydroxyindole acetic acid (5-HIAA), uric acid (UA) and dopamine (DA). The oxidation potential of the interferences is closed to or negative than that of 5-HT at bare conventional electrode (approximate oxidation potential is 0.2 V for AA, 0.35 V for 5-HT, 0.35 V for 5-HIAA, 0.3 V for UA, 0.2 V for DA, vs. Ag/AgCl) [10] and the concentration of the interferences is higher than that of 5-HT (for example, the normal ranges of UA in human serum is 120 - 380 μmol/L [11]). Another is the sensitivity because the normal level of 5-HT in human serum is 0.22 - 2.06 μmol/L. The third is the electrode passivation/biofouling caused by biomolecule adsorption from the oxidation products of 5-HT [12].
Much effort was done to solve the above-mentioned problems. For example, various polymer films, such as poly(phenosafranine) [13], Nafion films [14], and overoxidized polypyrrole (OPPy) [15, 16] were used to modify the electrode to reduce the interference. The OPPy film has received attention in the detection of cations because of its charge repulsion against anions and molecular sieve properties. Wightman's group fabricated overoxidized polypyrrole films modified carbon fiber microelectrodes for the selective detection of dopamine, in which the OPPy films attracted cations to increase sensitivity, excluded anions to reduce interference and prevented blockage of the electrode by larger molecules to reduce fouling of the electrode surface [16]. The overoxidation of polypyrrole can result in addition of carbonyl and carboxylic groups, which is promising for the selective detection of 5-HT. However, upon overoxidation of polypyrrole, the conductivity of OPPy on electrode is limited [17]. Therefore, it is still needed to develop selective and sensitive electrochemical method for the detection of 5-HT.
The aim of the work is to develop a selective and sensitive electrochemical method for the detection of 5-HT at microelectrode using nanoparticles-signal amplified strategy of gold nanoflowers (Au NFs) and high cation selection of overoxidized polypyrrole (OPPy). Au nanomaterials were used in this work because of their biological compatibility, high surface-to-volume ratio, excellent electronic conductivity and novel catalytic activity [18, 19]. Carbon fiber microelectrode (CFME) was used as base electrode because of its small size, enhanced mass transport, reduced ohmic voltage drops, low charging currents, and great signal-to-noise [20].
The scheme of fabrication of OPPy/Au NFs/CFME was shown in Scheme 1. Firstly, the gold nanoflowers were deposited on the carbon fiber by electroless deposition method with dipping carbon fiber and copper wire into chloroauric acid solution (HAuCl4). Using Cu wire as reducing agent, carbon fiber as supporter, AuCl4- was directly reduced to Au and loaded on the carbon fiber because the potential of AuCl4-/Au is much positive than the potential of Cu2+/Cu (AuCl4-/Au = + 1.002 V vs. SHE, Cu2+/Cu = + 0.337 V vs. SHE). During the electroless deposition process, the amaranth Cu wire became black and the black carbon fiber turned to yellow. The color change for Cu wire is ascribed to the presence of gold on Cu wire (Fig. S2 in Supporting information). The typical well-defined and reproducible cyclic voltammograms (CVs) were appeared at Au NFs/CFME in H2SO4 for 10 consecutive cycles with an oxidation peak at 1.30 V and a reduction peak at 0.93 V, suggesting that Au NFs are successfully modified onto carbon fiber and have good adhesion to the carbon fiber (Fig. S3A in Supporting information) [21]. Secondly, the pyrrole was repeatedly polymerized and overoxidized on the Au NFs modified CFME (Au NFs/CFME) by electrochemical method to form gold nanoflowers and overoxidized polypyrrole modified carbon fiber microelectrode (OPPy/ Au NFs/CFME). In the process of electrochemical polymerization, an oxidation peak was appeared at 0.80 V corresponding to the oxidation of pyrrole to obtain gold nanoflowers and polypyrrole modified carbon fiber microelectrode (PPy/Au NFs/CFME) [22] (Fig. S3B in Supporting information). The polypyrrole was further overoxidized in 0.5 mol/L NaOH to obtain OPPy/Au NFs/CFME. During the overoxidized process, the oxidization current eventually leveled off indicating complete overoxidation (Fig. S3C in Supporting information). The electrochemical behavior of the different electrodes was characterized in 1 mmol/L ferrocenecarboxylic acid (Fc-COOH) (Fig. S3D in Supporting information). Typical sigmoidal CVs were recorded for all modified electrodes. Compared to the bare CFME (curve a), the peaks current of FcCOOH at Au NFs/CFME (curve b) increased due to the good conductivity of Au NFs. The peaks current of Fc-COOH at PPy/Au NFs/CFME (curve c) further increased due to high electronic conductivity and fast kinetics ion exchange with the surrounding medium of porous polypyrrole film [23]. After polypyrrole was overoxidized (curve d), the current of Fc-COOH anion is partially suppressed due to the repulsion between Fc-COO- and the OPPy film [24]. These results indicate the successful fabrication of the OPPy/Au NFs/CFME.

|
Download:
|
| Scheme 1. Scheme of fabrication of OPPy/Au NFs/CFME. | |
The obtained different electrodes were characterized by field emission scanning electron microscopy (FE-SEM). Fig. 1 shows the high resolution and low-resolution FE-SEM images of Au NFs/CFME and OPPy/Au NFs/CFME. From the FE-SEM images, it can be clearly seen that trilateral nanocones structure and high density of Au NFs were formed onto carbon fiber surface (Figs. 1A and B). And the size of Au NFs is about 100–500 nm (Fig. 1A). The diameter of Au NFs/ CFME was increased to 9.0 ± 0.1 μm (n = 6, Fig. 1B), which is slightly larger than that of bare carbon fiber (7.8 ± 0.2 μm, n = 6, Fig. S1C in Supporting information). After encapsulation of overoxidized polypyrrole films onto Au NFs/CFME, the diameter of the OPPy/Au NFs/CFME was further slightly increased to 9.4 ± 0.2 μm (n = 6, Fig. 1H). Elemental mapping from FE-SEM showed that Au NFs were homogenously deposited onto the carbon fiber (Fig. 1E). There were the increasing densities of C, O and N elements on OPPy/Au NFs/CFME (Figs. 1I, J and L), further confirming that the overoxidized polypyrrole was deposited successfully on Au NFs/CFME.

|
Download:
|
| Fig. 1. High-resolution (A, G), low-resolution (B, H) FE-SEM images and the corresponding EDX spectrum elemental color maps (C (C, I), O (D, J, Au (E, K), N (F, L)) of the Au NFs/CFME (A–F) and OPPy/Au NFs/CFME (G–L). | |
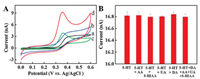
Electrochemical behavior of 5-HT in 0.1 mol/L phosphate buffer saline (PBS, pH 7.0) at different electrodes was investigated and the resulting CVs were shown in Fig. 2A. It can be seen that a sluggish and weak irreversible oxidation wave of 5-HT was appeared at 0.404 V at bare CFME (curve a). The peak oxidation current was increased to 2.155 nA and 3.537 nA, and the peak oxidation potential was negative shifted to 0.391 V and 0.367 V at Au NFs/ CFME (curve b) and overoxidized polypyrrole modified carbon fiber microelectrode (OPPy/CFME, curve c), respectively. At OPPy/ Au NFs/CFME (curve d), the oxidation current was increased to 8.264 nA and the peak potential was negative shifted to 0.364 V. The increase of current and negative shift of oxidization peak potential may be attributed to the composite effect of the larger surface area and good electrochemical conductivity of Au NFs and preconcentration of cations by OPPy [24]. Therefore, OPPy/Au NFs/ CFME was chosen as modified electrode for 5-HT detection.
|
Download:
|
| Fig. 2. (A) CVs of 5 μmol/L 5-HT in 0.1 mol/L PBS (pH 7.0) at bare CFME (a), Au NFs/ CFME (b), OPPy/CFME (c) and OPPy/Au NFs/CFME (d). Scan rate: 10 mV/s. (B) DPV peak currents of 5 μmol/L 5-HT in the absence and presence of interferences at OPPy/Au NFs/CFME (data from Fig. S4). | |
To illustrate the good electrochemical properties of OPPy/Au NFs/CFME toward the oxidation of 5-HT, DPV peak currents of 5-HT at OPPy/Au NFs/CFME in the absence and presence of different interferences, including AA, 5-HIAA, UA and DA, were compared in Fig. 2B (data from Fig. S4 in Supporting information). It can be seen that there is no interference for the detection of 5-HT (DPV signal change < 4%) even in the presence of 200-fold AA, 10-fold 5-HIAA and 10-fold UA, further confirming the charge repulsion properties against anions of OPPy/Au NFs/CFME. No obvious interference for the detection of 5-HT was obtained in the presence of 2-fold DA because of the good peak separation at OPPy/Au NFs/CFME (Figs. S4D and E). Good permselectivity and effective detection of 5-HT with neglected interference from AA, 5-HIAA, UA and DA are obvious.
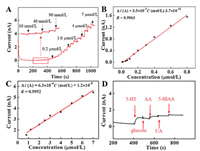
The analytical performance for the detection of 5-HT was checked by amperometric i–t curve method using different electrodes at optimized potentials. 0.6 V was chosen as optimized potential at bare CFME (Fig. S5A in Supporting information). 0.4 V was optimized potential at Au NFs/CFME (Fig. S5B in Supporting information) and OPPy/Au NFs/CFME (Fig. S5C in Supporting information). The oxidation currents increased gradually with the increase of 5-HT concentration from 10 nmol/L to 7.0 μmol/L with a detection limit (DL) of 2.3 nmol/L (S/N = 3) at OPPy/Au NFs/CFME (Fig. 3). Compared with the analytical performance for 5-HT at CFME and Au NFs/CFME (Fig. S6 in Supporting information), the OPPy/Au NFs/CFME showed higher sensitivity (3.5 × 10-3 A L mol-1) and lower detection limit (2.3 nmol/L) than that at bare CFME (sensitivity: 5.7 × 10-4 A L mol-1, DL: 15 nmol/L) and Au NFs/CFME (sensitivity: 2.1 × 10-3 A L mol-1, DL: 78 nmol/L). Compared with other electrochemical methods for the detection of 5-HT (Table S1 in Supporting information), the fabricated OPPy/ Au NFs/CFME showed lower detection limit with a reasonable linear range. The selectivity was examined for 5-HT detection (Fig. 3D). The first injection of 5 μmol/L 5-HT produced a remarkable increase in the current while the subsequent addition of 0.5 mmol/L glucose, 0.5 mmol/L AA, 50 μmol/L UA and 50 μmol/L 5-HIAA only produced negligible responses. Excellent selectivity toward 5-HT detection is obtained. Finally, we detected the concentration of 5-HT in diluted human serum samples. The recoveries were found in the range of 94.5%-102.3% (Table S2 in Supporting information), which indicated the feasible application for 5-HT detection in real samples using OPPy/Au NFs/CFME.
|
Download:
|
| Fig. 3. (A) Amperometric responses to successive additions of different concentrations of 5-HT in 0.1 mol/L PBS (pH 7.0) with an applied potential of 0.4 V at OPPy/ AuNPs/CFME. (B, C) Calibration curve of 5-HT. (D) Amperometric response to successive addition of 5 μmol/L 5-HT, 0.5 mmol/L glucose, 0.5 mmol/L AA, 50 μmol/L UA and 50 μmol/L 5-HIAA in 0.1 mol/L PBS (pH 7.0) with an applied potential of 0.4 V at OPPy/AuNPs/CFME. | |
It is widely appreciated that 5-HT electrooxidation can lead to rapid electrode passivation/fouling caused by adsorption of the oxidation products of 5-HT on working electrode surface [25]. Thus, the variation of current response of 5-HT at bare CFME and OPPy/Au NFs/CFME was studied by recording consecutive CVs with 1 min intervals between each CV (Fig. S7 in Supporting information). The initial CV shows the highest current responses and subsequent cycles show decrease trend of the current responses. For CFME, ip decreases to 42% of the initial value after 5 cycles (Figs. S7A and C). For OPPy/Au NFs/CFME, ip decreases to 71% of the initial value after 5 cycles (Figs. S7B and C). OPPy/Au NFs/ CFME shows better resistance toward electrode surface passivation/fouling than CFME.
In conclusion, we have fabricated OPPy/Au NFs/CFME using electroless deposition and electrochemical method and developed a highly selective and sensitive electrochemical method for the detection of 5-HT. The OPPy/Au NFs/CFME exhibits a wide linear range, low detection limit, good selectivity and anti-fouling performance in the detection of 5-HT because of the incorporation of larger surface area and electrochemical conductivity of Au nanomaterials with cation selective property of overoxidized polypyrrole. We successfully applied the modified electrode for the detection of 5-HT in human serum samples. The developed electrochemical method, incorporating signal amplification of Au NFs with higher cation selection of OPPy, provides a promising tool for the detection of 5-HT in biological systems. The combination of electroless deposition and electrochemical methods for the fabrication of OPPy/Au NFs/CFME is simple and less timeconsuming, which offers great promise for electrode modification and sensing applications in microelectrode.
AcknowledgmentsWe thank the National Natural Science Foundation of China (Nos. 21775097 and 21804106), the China Postdoctoral Science Foundation (No. 2017M620444) and the Fundamental Research Funds for the Central Universities (Nos. XJJ2018247 and GK201801006).
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.05.042.
| [1] |
X. Wang, D.Z. Liu, A.J. Li, Chin. Chem. Lett. 19 (2008) 40-42. DOI:10.1016/j.cclet.2007.11.020 |
| [2] |
P.A. Robiolio, V.H. Rigolin, J.S. Wilson, et al., Circulation 92 (1995) 790-795. DOI:10.1161/01.CIR.92.4.790 |
| [3] |
Z.D. Peterson, M.L. Lee, S.W. Graves, J. Chromatogr. B 810 (2004) 101-110. DOI:10.1016/S1570-0232(04)00597-5 |
| [4] |
J. Chauveau, V. Fert, A.M. Morel, et al., Clin. Chem. 37 (1991) 1178-1184. DOI:10.0000/PMID1855288 |
| [5] |
T.J. Panholzer, J. Beyer, K. Lichtwald, Clin. Chem. 45 (1999) 262-268. DOI:10.0000/PMID9931050 |
| [6] |
J.P. Danaceau, G.M. Anderson, W.M. McMahon, et al., J. Anal. Toxicol. 27 (2003) 440-444. DOI:10.1093/jat/27.7.440 |
| [7] |
G. Curzon, A.R. Green, Brit. J. Pharmacol. 39 (1970) 653-655. DOI:10.1111/j.1476-5381.1970.tb10373.x |
| [8] |
N.W. Barnett, B.J. Hindson, S.W. Lewis, Anal. Chim. Acta 362 (1998) 131-139. DOI:10.1016/S0003-2670(98)00058-0 |
| [9] |
A. Abdalla, C.W. Atcherley, P. Pathirathna, et al., Anal. Chem. 89 (2017) 9703-9711. DOI:10.1021/acs.analchem.7b01257 |
| [10] |
D.L. Robinson, A. Hermans, A.T. Seipel, et al., Chem. Rev. 108 (2008) 2554-2584. DOI:10.1021/cr068081q |
| [11] |
D. Lakshmi, M.J. Whitcombe, F. Davis, et al., Electroanal. 23 (2011) 305-320. DOI:10.1002/elan.201000525 |
| [12] |
J. Li, Z. Peng, E. Wang, J. Am. Chem. Soc. 140 (2018) 10629-10638. DOI:10.1021/jacs.8b01302 |
| [13] |
T. Selvaraju, R. Ramaraj, Electrochem. Commun. 5 (2003) 667-672. DOI:10.1016/S1388-2481(03)00151-6 |
| [14] |
L.Q. Xie, Y.H. Zhang, F. Gao, et al., Chin. Chem. Lett. 28 (2017) 41-48. DOI:10.1016/j.cclet.2016.05.015 |
| [15] |
C.C. Hsueh, A. Brajter-Toth, Anal. Chem. 66 (1994) 2458-2464. DOI:10.1021/ac00087a009 |
| [16] |
K. Pihel, Q.D. Walker, R.M. Wightman, Anal. Chem. 68 (1996) 2084-2089. DOI:10.1021/ac960153y |
| [17] |
F. Beck, P. Braun, M. Oberst, Ber. Bunsenges. Phys. Chem. 91 (1987) 967-974. DOI:10.1002/bbpc.19870910927 |
| [18] |
Y.L. Chen, G.W. Zhao, L. Lin, Chin. Chem. Lett. 29 (2018) 1633-1636. DOI:10.1016/j.cclet.2018.01.055 |
| [19] |
Y.X. Lai, C.X. Zhang, Y. Deng, et al., Chin. Chem. Lett. 30 (2019) 160-162. DOI:10.1016/j.cclet.2018.07.011 |
| [20] |
Z. Lin, Y. Takahashi, Y. Kitagawa, et al., Anal. Chem. 80 (2008) 6830-6833. DOI:10.1021/ac801389d |
| [21] |
K.Q. Wang, X. Zhao, B. Li, et al., Anal. Chem. 89 (2017) 8683-8688. DOI:10.1021/acs.analchem.7b02814 |
| [22] |
P.K. Sharma, G. Gupta, V.V. Singh, et al., Synthetic Met. 160 (2010) 2631-2637. DOI:10.1016/j.synthmet.2010.10.016 |
| [23] |
S. Shahrokhian, R.S. Saberi, Electrochim. Acta 57 (2011) 132-138. DOI:10.1016/j.electacta.2011.04.029 |
| [24] |
J. Li, X.Q. Lin, Sens.. Actuat. B-Chem. 124 (2007) 486-493. DOI:10.1016/j.snb.2007.01.021 |
| [25] |
A.N. Patel, S.Y. Tan, T.S. Miller, et al., Anal. Chem. 85 (2013) 11755-11764. DOI:10.1021/ac401969q |
 2019, Vol. 30
2019, Vol. 30 

