Electrochemiluminescence (ECL) plays an important role in many biosensor technologies. As a kind of high-energy electron transfer process generated in the surface of the electrode, formed during the transfer of high-energy electrons, ECL is the perfect combination of electrochemistry and spectroscopy, the ECL-based sensor has many unique characteristics [1]. The sensor (or transducer) can sense information and transform the sensed information convert to electrical signals or other signal outputs, which can meet the requirements of information transmission, processing, storage, display, recording and control. Biosensor is a new method that can convert the concentration of analytes into other signals and detect them by combining biological sensitive elements and physical conduction technologies [2]. Biosensor is a device that uses a simple method to transmit biometric signals and physical signals, which consists of many identification components, such as voltage signals, optical signals, electrochemical signals, etc. [3]. At present, biosensors have been widely developed in various aspects and have brought enormous challenges to the early diagnosis of diseases [4]. Biosensors must have the characteristics of good stability, fast response, high sensitivity and simplicity, to meet the requirements of commercialization and clinical application. ECL biosensor is a method based on detecting the interaction between biometric unit and corresponding target, achieving quantitative analysis of the target by detecting an ECL signal change caused by an ECL active substance.
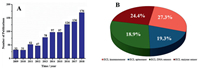
The electrochemical biosensor is mainly composed of two parts: a biomolecule recognition component and a signal conversion component. The biomolecular recognition component is composed of biosensitive membrane having molecular recognition function (such as enzyme, microorganism, tissue, antibody antigen, nucleic acid and cell) for detecting whether a sample contains a substance to be tested [5]. Check out the number of published literature in the field of electrochemiluminescence biosensors trends during nearly a decade (Fig. 1A), we can found that the numbers of articles published in the field of ECL biosensors were small and growing slowly from 2009 to 2012, while the number increases year by year, and there is a trend of continued increase from the year 2013 until 2018. It is indicated that ECL biosensor was still a hot research fields in the current. ECL biosensors are mainly divided into four types: ECL immunosensors, ECL aptamer sensors, ECL DNA sensors, and ECL enzyme sensors. Taking the number of articles published in 2009–2018 as an example, it can be seen (Fig. 1B) that among the four types of sensors, the proportion of immunosensors and enzyme sensors was slightly larger, and the other two publications were equivalent in number. In general, four sensors are in a balanced development stage.
|
Download:
|
| Fig. 1. (A) The number of published literatures in the field of electrochemical biosensors from 2009 to 2018; (B) Distribution of literatures of different types of biosensors published in 2009-2018. The data come from Web of Science. | |
Introducing nanomaterials into ECL sensing system, has brought revolutionary effect for improving sensitivity and application range of ECL sensing. Nanomaterials are widely used in the development of biosensor systems, and will continue to promote the development and application of biosensor because of different sizes, morphologies and chemical compositions, excellent chemical and optical properties. Certain metal nanomaterials can be used to construct biosensor to improve sensing sensitivity if it can catalyze ECL-related reactions. Our group has some research work on the introduction of nanomaterials into chemiluminescence systems to build chemiluminescence sensors [6-8]. With the rapid development of nanomaterials and their unique properties including the unique electrical properties, large specific surface area, and good biocompatibility, many of them have been widely used in the field of biosensors and sensitive determination. Review articles on the field, which either were published some years ago or focused on other aspect, have been published before. Such as Wang Erkang group summarized the luminescent nanomaterial related ECL sensors, including semiconductor quantum dots (QDs), carbon-based nanomaterial, metal nanoclusters (NCs) and upconversion nanoparticles [9]. Lin's group summarized the characteristics and advantages of luminescence from metal NCs, and highlighted their applications in biomedicine [10]. Xu's group discussed recent vibrant developments in ECL, and highlighted novel ECL phenomena [1]. Zhu's group wrote a review which focused on developments in ECL assays during 2015–2016 [11]. This paper reviewed the recent advances in the application of ECL biosensor based on nanomaterials.
2. Electrochemical luminescence sensor based on nanomaterialsNanomaterials refer to materials that have at least one dimension in the H-dimensional space at a nanometer size (0.1–100 nm) or are composed of them as basic units, which is roughly equivalent to the scale of 10–100 atoms closely arranged together. Nanomaterials introduced into ECL sensing system, has brought revolutionary effect for improving sensitivity and application range of ECL sensor. Nanomaterials can effectively improve the biometric or physical signal transmission of biosensors in different ways. The application of nanomaterials in the field of ECL mainly includes the following aspects: nanomaterials used as ECL nucleus; nanomaterials for signal amplification; nanomaterials as ECL resonance energy transfer acceptors.
2.1. Nanomaterial used as ECL nucleusThe optical and electrochemical properties of quantum dots are related to size and material composition, and own a strong spectral absorption, narrow and symmetric emission spectrum, a high quantum yield, excellent light stability and resistance to photobleaching and other advantages. Current quantum dot-based electrochemical luminescence sensor was constructed based on the principle of quantum dots as electrochemical luminescence nucleus and co-reactants participate. Oxalate (C2O42-), persulfate (S2O82-), tripropylamine (TPA) or amine-related derivatives, H2O2 and dissolved oxygen are commonly used co-reactants [12]. ECL spectrum of quantum dot has a relatively common feature, that is, the spectrum is generally red-shifted compared to its fluorescence spectrum, what means that its excited state is different. Studies have shown that, compared to the effect of quantum dot size and surface ligand doping on fluorescence, the ECL intensity of quantum dots is more sensitive to surface chemistry and surface states. Therefore, researchers believe that the fluorescence of quantum dots is mainly from the interior, while the ECL depends more on the surface state [13]. There are many dangling bonds on the surface of the nanoparticles, which form many surface defect states. After the semiconductor nanocrystals are excited by light, the photo-induced carriers that are limited to the surface defect state will generate surface state luminescence at top speed. The smoother the surface of the quantum dot, the weaker the surface's ability to capture carriers and the weaker the surface state.
Since the quantum dot ECL sensor was first developed and successfully used in the detection of H2O2 by Ju huangxian group (Nanjing University) in 2004 [14], the ECL sensor based on quantum dot has received extensive attention due to its strong response signal, low detection limit, catalytic effect, strong antiinterference ability, and easy long-term detection, and has been used in different research fields by interacting with different nanomaterials and other detection instruments. For example, Yang et al. implemented high-sensitivity ECL aptamer sensors based on quantum dot and exonuclease-catalyzed cyclic amplification of target for ochratoxin (OTA) detection [12]. In addition, a series of nanocomposites have also been applied to the construction of aptamer sensors. For example: Shi et al. designed a simple, labelfree ECL aptasensor based on the ECL effect of cadmium sulfidegraphene (CdS-GR) nanocomposites, for the detection of carcinoembryonic antigen using persulfate as a coreactant [15].
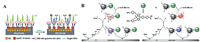
With the development of synthetic methods, a variety of quantum dots, such as complex, core-shell, oxide quantum dots, and single-element components, have been prepared, and used as luminescent nucleus for ECL sensor applications. Composite quantum dots have the advantages of simple synthesis steps and strong ECL, commonly used as ECL nucleus, including CdS [16], CdSe [17], CdTe [18], PbS [19] and so on, some of the construction schematic diagrams were shown in Fig. 2. Although quantum dots are widely used in the field of biosensor, they must also face the common problem of quantum dot toxicity [20]. At present, a large number of research work on how to face and solve the toxicity of quantum dots has been reported [21], such as: Liu et al. prepared cysteine-coated CdSe(CdZnS) QDs, whose excellent size demonstrates good kidney clearance and good biocompatibility [22]. Zhu et al. prepared a dual-signal amplified electrochemiluminescence (ECL) biosensor by inlaying the CdTe quantum dots (QDs) into the mesoporous silica (mSiO2) and future coating the surface with the silica layer. Silica shell would effectively prevent the leak of QDs to increase the biocompatibility [23]. It can be seen that there are mainly the following ways: (1) avoiding the use of small-sized quantum dots (below 10 nm); (2) using quantum dots without heavy metals and organic stabilizers as much as possible; (3) utilizing quantum dots modified with low toxicity functional groups such as carboxyl groups; (4) protection heavy metalcontaining quantum dots with ZnS or BSA as shells. There are more methods to combat quantum dot toxicity needed researchers to develop in future, making quantum dots a greater breakthrough in biotherapy in hope.
|
Download:
|
| Fig. 2. (A) Schematic illustration of QDs-based ECL DNA sensor. Copied with permission [18]. Copyright 2013, the Royal Society of Chemistry. (B) ECL mechanisms of PbS-BDY nanocrystals based on PbS. Copied with permission [19]. Copyright 2015, American Chemical Society. | |
2.2. Nanomaterials for signal amplification
Nanomaterials are used for signal amplification mainly through the following methods: nanomaterials as carriers, nanoparticles for electrode modification, nanomaterials as catalysts, multifunctional nanomaterials or nanocomposites.
Nanomaterials have a large specific surface area and can be used as a carrier loading small molecule luminescent reagent for the construction of highly sensitive ECL sensor. At present, nanomaterials as carriers are mainly metal materials (especially gold nanoparticles), magnetic nanoparticles and silicon nanoparticles. As an excellent small molecule carrier, gold nanoparticles have the advantages of simple synthesis, good biocompatibility, various sizes and convenient functions. The magnetic nanoparticles interact with an external magnetic field provide a method for separating samples from the suspension efficiently, and the magnetic nanoparticles also have the advantages of simple preparation and unique magnetic properties. Meanwhile, silicon nanoparticles are considered as another common carrier for ECL, due to the advantages of large specific surface area, low toxicity, and low price. In addition, the silicon nanoparticles can protect the ECL nucleus from the external environment by doping, thereby improving stability and ECL intensity. Lin et al. [24] split the aptamer into two fragments, one ofwhichwasimmobilizedon the surfaceof thegoldelectrodeby the Au-S bond and the other was functionalized by Ru(bpy)32+-doped silicon nanoparticle (Ru-SNPs), as shown in Fig. 3A. In the presence of thrombin, the two segments of the aptamer can be combined to form a G-tetramer structure that allows Ru-SNPs to attach to the surface of the gold electrode to enhance ECL signal with a detection limit of 0.2 pmol/L. One of the current research work of our group is about using silicon nanoparticles as the carrier of luminescent nucleus. We synthesized a porous hollow silica sphere nanomaterial with special morphology and used it to load Ru(bpy)32+ as luminescence core, which greatly enhances the luminescence signal compared to the conventional morphological silicon nanoparticles.

|
Download:
|
| Fig. 3. (A) Schematic illustration of the dual signal amplification strategy-based ECL aptasensor for detection of thrombin. Copied with permission [24]. Copyright 2014, American Chemical Society. (B) Schematic illustration of visual sensing. Copied with permission [26]. Copyright 2014, the Royal Society of Chemistry. (C) Schematic illustration of ECL biosensor for detection of PrV antibody. Copied with permission [28]. Copyright 2014, American Chemical Society. (D) Schematic illustration of the ECL immunosensor preparation process and the possible luminescence mechanism. Copied with permission [29]. Copyright 2016, Elsevier. | |
Electrode modification is the most important step in the construction of ECL biosensor platforms. Preparation of an electrode modification interface that is stable, highly efficient, biocompatible, and has good signal amplification effect is of great significance for the successful construction of a highly sensitive biosensor system. Currently, nanoparticles having different compositions, sizes, shapes, surface properties, and functions have been used for electrode modification. Gold particles can not only be used as an ECL carrier to increase the amount of electroluminescent materials, but also a good electrode modification material, which can be used to increase the electron transfer at the electrode interface. To this end, various chemically modified electrodes have been prepared to improve the analytical performance of the assay [25]. Recently, Au nanoparticle modified electrodes have been used in a large number of different ECL sensing systems, and both have enhanced ECL and increased detection sensitivity [26], which was shown in Fig. 3B. The special properties of carbon materials in terms of electronics, chemistry, mechanics and structure have made them an attractive electrode modification material. Carbon nanotubes not only have superconducting or semiconducting properties, but also have hollow cavities that can store certain small molecules. In addition, carbon nanotubes can be easily functionalized, modified by other functional groups, thereby can better adhere to biomolecules. Currently, biosensor can be constructed by immobilizing suitable biomolecules on carbon nanotubes. The other application of carbon nanotubes in biosensor is based on its good ability to accelerate electron transfer. The current of the Ru(bpy)32+/TPA ECL reaction system was accelerate in the surface of carbon nanotube modified electrode compared with the unmodified electrode [27]. Graphene, as another star carbon material, has strong mechanical strength, large specific surface area, excellent electrical conductivity and thermal conductivity, and has become another increasingly popular electrode modification material. Shao et al. [28] constructed a biosensor based on gold nanoparticlesgraphene (Au-GN) nanocomposites, luminescent reagent functionalized silica nanoparticles and biotin-avidin (B-SA), achieved three times or even multiple times of signal amplification without any other media catalysis, schematic was shown in Fig. 3C.
Yuan [29] constructed a sandwich-type ECL immunosensor platform based on the prepared functionalized nanocomposites as a carrier immobilized secondary antibody and successfully used it for the sensitive determination of the tumor marker human mucin (MUC1), the schematic was shown in Fig. 3D. Finally, the ECL immunosensor achieves a wide detection range of 10 fg/mL to 10 ng/mL, completes the effective determination of the target in clinical serum samples in the meantime, and also has potential applications for biological analysis of other protein markers.
Certain metal nanomaterials can catalyze ECL-related reactions, what can improve sensitivity during constructing biosensor. With the development of biosensor technology, features a single nanomaterial cannot meet the needs of ultra-sensitive biosensor. Therefore, multifunctional nanocomposites composed of variety of nanomaterials are becoming more and more extensive used in the field of biosensor.
2.3. Nanomaterials as ECL resonance energy transfer receptorsAt the outset, resonance energy transfer (RET) described nonradiative energy transfer between two fluorophores [30]. Recently, the phenomenon of resonance energy transfer excited by ECL (ECLRET) has attracted more and more attention with the development of ECL technology, especially the microanalysis of nucleic acids, proteins, small molecules and the like. Compared with the earliest fluorescence mode, ECL-RET does not require external excitation sources, no scattered light interference, so, there are more advantages in the construction and application of biosensors [31].
Researchers have developed a series of biosensor methods for the determination of small molecules and cells based on the principle of ECL-RET. In the ECL-RET system, it is the key to find the right electrochemical luminescent donor and the receptor with a good spectral overlap. Obviously, quantum dots are an excellent ECL donor due to their excellent optical properties. As luminescent acceptors, various materials, including quantum dots themselves, can also serve as donor-acceptor pairs for the construction of ultrasensitive ECL-RET sensor systems. In 2004, Wargnier et al. reported that RET can occur between CdSe/ZnS quantum dots which with oppositely charged [32]. What is worth noticing is that ECL-RET model in intramolecular, which contains both energy donors and energy receptors in one same molecule, can improve effective collision efficiency and reduce energy loss caused by space barriers to achieve high-intensity ECL response and high output ECL signal. Wang et al. [33] synthesized a novel molecule Ru (bpy)2(mcbpy)2+-PEI-ABEI by PEI as an intermediary, and constructed an ECL immunosensor with high signal response to trace concentrations of Col Ⅳ antigen based on this. Wherein, ABEI as a donor promoted the ECL intensity of the receptor by way of energy transfer, and realized the intramolecular resonance energy transfer amplification.
3. Application of ECL biosensor in analysisECL biosensors are mainly divided into two parts of biomolecular recognition components and signal conversion components. ECL biosensors can be mainly classified into immunosensor, aptamer sensor, DNA sensor and enzyme sensor depending on the biorecognition molecule.
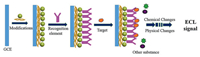
3.1. ECL immunosensorElectrochemical immunosensor is a new type of biosensor which is constructed by combining immunoassay with electrochemical sensing technology and was applied to the analysis of trace immunogenic substances, has the characteristics of good specificity, high sensitivity, short time consumption and trace determination. It has important application value in the fields of disease diagnosis, food hygiene and environmental testing. The working principle of the immunosensor is a solid phase immunoassay, which is similar to the traditional immunoassay in that an antigen or antibody is immobilized on the surface of a solid support to detect antigens or antibodies in the sample. The construction principle of the immunosensor is shown in Fig. 4.
|
Download:
|
| Fig. 4. Scheme of the principle for immunosensor. | |
According to the reaction structure between antigen and antibody, the construction mode of immunosensor can be divided into direct method, double antibody sandwich method and competition method. The so-called direct method is based on a single immune reaction between the antigen and the antibody, immobilizing one of the classes on a solid substrate and then directly capture the other, immediately following monitoring the change of the signal to quantitatively analyze the content of the target. The method is easy to operate and has a fast response speed, but the analysis sensitivity is low. For example, Jie et al. [34] used the nano-composite layer CdSeNCs/CNT-CHIT to immobilize antibody and directly capture antigen by immunoreaction. The higher the concentration of antigen in the detection substrate, the greater the hindering effect of the formed immune complex on the electron transport of the electrode. An ECL sensor which based on this negative correlation linear relationship was prepared for immunoglobulin detection.
The double-antibody sandwich type immunoreaction has one more time antigen-to-antibody recombination than the direct method, forming a sandwich structure of the anti-target antigensecondary antibody. The greater the concentration of the target, the more secondary signal-labeled secondary antibodies can be combined, so the higher the detection signal. Based on this positive correlation linearity, this type of immunosensor has the advantages of low background signal and high sensitivity. For example, Tian et al. [35] used electrode modified by CD-NGs loaded primary antibody, immobilized secondary antibody with Ni Au Pt-NGs nanocomposite, then constructed a signal-enhanced type electrochemical sensors by sandwich immunoreaction for carcinoembryonic antigen sensitive detection.
The ECL immunosensor combines the high sensitivity of ECL technology with the high selectivity of immunological analysis, and has an incomparable advantage for most protein-based targets [36]. The immune complex formed by the specific recognition of the antigen and the antibody can cause a change in the ECL response signal, thereby achieving the purpose of quantitative analysis of the target protein.
3.2. ECL aptamer sensorAptamer is a single-stranded DNA or RNA fragment screened by the systematic evolution of ligands by exponential enrichment (SELEX) [37]. The first research report of aptamer in the ECL was used to detect anthrax spores, cholera toxin and staphylococcal enterotoxin B in 1999. ECL aptamer sensors have developed rapidly in recent years, have made great progress in detection, and developed various aptamer-based ECL biosensor formats for the determination of many targets including proteins and small molecules. When constructing these biosensors, the identified aptamers are first immobilized on the electrode surface, and signals from electrochemical (EC) or ECL active probes are used to indicate the mixing process. Therefore, the fixation of the aptamer and the introduction of the probe play an important role in the performance of an aptamer sensor. Fig. 5 shows the basic configuration ECL biosensor basic principles and specific.

|
Download:
|
| Fig. 5. Scheme of the principle for aptasensor. | |
To construct an aptasensor, something to be done first is to modify the DNA or DNA-labeled substance to the electrode interface or carrier. The immobilization of the probe in practical applications depends on different interfaces. Currently, the most commonly used aptamer immobilization techniques are covalent coupling, self-assembly, and avidin-biotin. The covalent coupling method is a surface activation using a functional group or a coupling activator to covalently couple the reactive group at the end of the aptamer probe with some reactive groups on the surface of the electrode, such as -COOH, –NH, -OH, and the like. For example, Jiang et al. [38] immobilized ABEI on a -COOH-labeled hairpin probe by an amide reaction, luminescence was captured onto the electrode surface using a protein-aptamer complexdriven three-dimensional DNA nanomachine signal probe, the ECL biosensor was constructed base on this to realize the quantitative analysis of mucin 1 (MUC1). Liu et al. [39] prepared an autocatalytic PTC-luminol complex by amide reaction of luminol with perylenetetracarboxylic acid (PTCA), and prepared secondary antibody complex by using composite nanomaterial prepared as carrier of secondary antibody. Finally, an intelligent ECL immunosensor was built based the sandwich-type immune response patterns between the primary antibody, secondary antibody and antigen complex, realized the sensitive determination of N-terminal probrain natriuretic peptide (NT-pro BNP). The self-assembly method utilizes the self-assembly of molecules, and generally uses gold electrodes or gold nanoparticles or deposits gold (Dp Au) on the electrodes as a substrate, and fixing with Au-S bonds. For example, Herne [40] reported immersing the gold electrode in the SH–DNA solution and continuing to incubate in the decylhexanol solution in order to blocking the remaining non-specific binding sites on the electrode surface to prevent non-specific adsorption of SH–DNA, making the monolayer of DNA molecules assembled to the surface of the gold electrode more orderly. As far as known, the interaction between biotin-avidin is the most intensive non-covalent effect, and a multi-level signal amplification system that significantly improves detection sensitivity and now widely used to construct sensors. For example, Chai et al. [41] directly modified streptavidin-coated Au nanoparticles (Au NPs) onto the electrode, and the aptamer of the two substances functionalized by biotin was immobilized by the interaction between avidin and biotin, for sensitive detection of adenosine and thrombin. Li et al. [42] reported a "sandwich" immunosensor that labeled the Fe3O4@APTES complex with electrocatalytic properties with biotin, immobilized biotinylated antibodies on the electrode surface and bound to avidin molecules. According to the principle of one avidin and four biotin functions, the process of "biotinavidin-biotin" was repeated to achieve multiple amplification effects and achieve ultra-sensitive detection of the target.
3.3. ECL DNA sensorDNA is the basic genetic material of all cellular organisms and can constitute genetic instructions, plays the role of storage and genetic information. Therefore, the study of DNA is of great significance in exploring the origin of life and studying the evolution of species and seeking treatment. Sequence identification and detection are usually carried out by hybridization analysis, which is one of the most widely used techniques in the field of biochemistry research and molecular biology research in recent years, also one of the powerful tools for qualitative identification and detection of specific sequence molecules. Fig. 6 shows the basic construction principle of the DNA sensor. Bard et al. [43, 44] studied the interaction of DNA molecules with RuBpy and Osmium pyridine by ECL, which laid a foundation for the application of ECL in biological DNA analysis. An ECL DNA biosensor is a method in which a single-stranded DNA probe is immobilized on the surface of electrode, and the electrode acts as a signal transducer to derive a hybridization reaction occurring on the surface. Since most of the time is electrochemically inactive, it is necessary to add electrochemically active recognition components to the electrochemical detection, which can selectively interact with doublestranded DNA or single-stranded DNA usually, so DNA biosensors analyze hybridization reactions by detecting signals from these electrochemically active components typically.

|
Download:
|
| Fig. 6. Scheme of the principle for DNA sensor. | |
Rusling et al. [45] assembled cationic polymer film and DNA molecules on the surface of electrode layer by layer, and then realized recognition and detection of targets by ECL by using catalysis of polymer membranes on DNA. Liu [46] et al. developed a novel multiplex ECL DNA sensor for determination of hepatitis B virus (HBV) and hepatitis C virus (HCV) based on multicolor CdTe quantum dots (CdTe QDs) and Au NPs. Under the optimum conditions, the ECL intensity of CdTe QDs551 and CdTe QDs607 and the concentration of target DNAHBV and target DNAHCV have good linear relationship in the range of 0.0005-0.5 nmol/L and 0.001–1.0 nmol/L respectively, and the limit of detection were 0.082 pmol/L and 0.34 pmol/L respectively (S/N = 3).
3.4. ECL enzyme sensorAs a special organic macromolecule, the enzyme has catalytic activity and high selectivity. Electrochemical enzyme sensors have the advantages of easy operation, low cost, fast analysis, good selectivity, high sensitivity, and can achieve on-site and real-time analysis and inspection, etc., and are more and more widely used in the fields of clinical diagnosis, food analysis, environmental monitoring and industry.
In 1962, Clark [47] first proposed the idea and principle of enzyme sensor. Subsequently, Updike et al. [48] combined glucose oxidase with an oxygen electrode to obtain the first enzyme sensor in 1967. To date, glucose oxidase is still widely used in enzyme biosensors because relatively high selectivity, stability and sensitivity compared to other types of enzymes, and is called "optimal enzyme" by Wilson et al. [49]. Recent years, researchers have been working on the third generation of enzyme sensors to achieve direct electron transfer between electrodes and enzymes to obtain sensors with better sensitivity and selectivity. However, because the active center of the enzyme is embedded in the thick insulating protein, the direct electron transfer between the enzyme and the electrode is somewhat hindered, which poses a great problem for researchers. Thus, a series of studies have been carried out to explore functionalized materials for promoting electron transfer between enzymes and electrodes, such as nanometals and their oxide materials [50, 51], room temperature ionic liquids [52], sol gels [53] and conductive polymer [54], etc. Graphene has been setting off a new research boom of graphene based enzyme sensor due to large specific surface area and good electron transfer capability since was discovered, which is expected to produce new types of composite materials with excellent performance to improve sensor sensing performance. For example, Chen et al. [55] prepared GO by using the Hummers' method, and then reduced it with hydrazine hydrate to obtain RGO. Dispersed the RGO in Nafion (Nf) and blend it with GOD to obtain a mixture of RGO-Nf-GOD, which was finally modified to the surface of the glassy carbon electrode to make an ECL sensor for glucose detection. Studies showed that GOD maintained good biological activity after immobilization on the composite membrane, and had a linear response to glucose of 2–100 μmol/L with a detection limit of 1 μmol/L and excellent stability.
3.5. OthersIn addition to the immnunosensors, aptamer sensors, DNA sensors, and enzyme sensors, the types of ECL biosensor also include cell sensor, microbial sensor, tissue sensor, and polypeptide sensor. For example, Yang et al. fabricated a single-cell sensor with a spatial architecture, and the ECL response of the sensor was applied to evaluate the validity of nanoprobe labeling [56]. Yuan Ruo et al. used the properties of target protein can specifically cleave polypeptide chains, constructed a polypeptide sensor, and achieved highly sensitive detection of prostatespecific antigens with positively charged gold nanoparticles as signal enhancers [57].
4. Prospects and developmentECL biosensor not only has high specificity recognition function, but also has the advantages of simple structure, low cost, high sensitivity, good selectivity, fast response, easy operation and low cost. It is widely used in the detection of disease-related nucleic acid molecules, proteins and functional small molecules, and attracted widespread attention of researchers.
This paper reviews the advances in the application of ECL biosensor based on nanomaterials in the past 10 years, which have been widely used in basic research and biomedical diagnostics. Nanotechnology made an important complement to the analysis and diagnostics (for example, the combination of new nanomaterials and aptamers makes aptamer sensors highly sensitive and selective). On the other hand, nanomaterials have a large impact on the signal amplification and sensitivity of ECL biosensors. Functionalized nanomaterials are currently a hot research field, nanoluminescence refers to the modification of luminescent reagents to the surface of nanomaterials through rational design, which not only maintains the inherent properties of nanomaterials, but also gives them new chemiluminescence activity. This material can be used not only as a nano-analytical probe, but also as a luminescence analysis interface that integrates multiple functions, thereby developing a new method of luminescence analysis, which has great potential for application in bioanalysis.
But beyond all that, look around, we found that not much achievement can really commercialized, although electrochemical, the traditional discipline, is playing a more important role. Therefor, how to make the sensor portable and get accurate analysis results will be the development direction of ECL biosensor on the commercial road. Go even further, how to improve the portability and accuracy of equipment is also the common development direction of other biosensors.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21675178, 21575167 and 21775167), the Guangdong Provincial Natural Science Foundation of China (No. 2016A030313358), the Research and Development Plan for Key Areas of Food Safety in Guangdong Province of China (No. 2019B020211001), and the Guangzhou Science and Technology Program of China (No. 201604020165), respectively.
| [1] |
Z.Y. Liu, W.J. Qi, G.B. Xu, Chem. Soc. Rev. 44 (2015) 3117-3142. DOI:10.1039/C5CS00086F |
| [2] |
P. Skládal, TrAC-Trend. Anal. Chem 79 (2016) 127-133. DOI:10.1016/j.trac.2015.12.009 |
| [3] |
P.F. Turner Anthony, Chem. Soc. Rev. 42 (2013) 3184-3196. DOI:10.1039/c3cs35528d |
| [4] |
M. Hasanzadeh, N. Shadjou, TrAC-Trends Anal. Chem. 80 (2016) 167-176. DOI:10.1016/j.trac.2015.07.018 |
| [5] |
N.J. Ronkainen, H.B. Halsall, W.R. Heineman, Chem. Soc. Rev. 39 (2010) 1747-1763. DOI:10.1039/b714449k |
| [6] |
R.K. Zhang, Y.F. Hu, G.K. Li, Anal. Chem. 86 (2014) 6080-6087. DOI:10.1021/ac5012359 |
| [7] |
R.K. Zhang, W.T. Huang, G.K. Li, Y.F. Hu, Anal. Chem. 89 (2017) 3353-3361. DOI:10.1021/acs.analchem.6b03898 |
| [8] |
W.T. Huang, Y.F. Hu, Z.Y. Lu, R.K. Zhang, G.K. Li, Microchim. Acta 185 (2018) 531. DOI:10.1007/s00604-018-3057-2 |
| [9] |
Q.F. Zhai, J. Li, E.K. Wang, ChemElectroChem 4 (2017) 1639-1650. DOI:10.1002/celc.201600898 |
| [10] |
Y. Su, T.T. Xue, Y. Liu, J.X. Qi, Z.K. Lin, Nano Res. 1 (2019) 1-15. |
| [11] |
L.L. Li, Y. Chen, J.J. Zhu, Anal. Chem. 89 (2017) 358-371. DOI:10.1021/acs.analchem.6b04675 |
| [12] |
J.P. Lei, H.X. Ju, TrAC-Trends Anal. Chem. 30 (2011) 1351-1359. DOI:10.1016/j.trac.2011.04.010 |
| [13] |
N. Myung, Y. Bae, A.J. Bard, Nano Lett 3 (2003) 1053-1055. DOI:10.1021/nl034354a |
| [14] |
G.Z. Zou, H.X. Ju, Anal. Chem. 76 (2004) 6871-6876. DOI:10.1021/ac049012j |
| [15] |
D. Xu, Z.L. Gao, N. Li, K.A. Li, Chin. Chem. Lett. 18 (2007) 561-564. DOI:10.1016/j.cclet.2007.03.010 |
| [16] |
J.J. Miao, T. Ren, L. Dong, Small 1 (2005) 802-805. DOI:10.1002/smll.200500072 |
| [17] |
Y.D. Wu, W.P. Peng, Q. Zhao, J.F. Piao, Chin. Chem. Lett. 28 (2017) 1881-1884. DOI:10.1016/j.cclet.2017.07.026 |
| [18] |
S.Y. Deng, L.X. Cheng, J.P. Lei, Nanoscale 5 (2013) 5435-5441. DOI:10.1039/c2nr33471b |
| [19] |
M. Hesari, K.N. Swanick, J.S. Lu, J. Am. Chem. Soc. 137 (2015) 11266-11269. DOI:10.1021/jacs.5b07633 |
| [20] |
V.L. Colvin, Nat. Biotechnol. 21 (2003) 1166-1170. DOI:10.1038/nbt875 |
| [21] |
A.M. Derfus, W.C.W. Chan, S.N. Bhatia, Nano Lett. 4 (2004) 11-18. DOI:10.1021/nl0347334 |
| [22] |
W. Liu, H.S. Choi, J.P. Zimmer, J. Am. Chem. Soc. 129 (2007) 14530-14531. DOI:10.1021/ja073790m |
| [23] |
H.Y. Zhu, S.N. Ding, Biosens. Bioelectron. 134 (2019) 109-116. DOI:10.1016/j.bios.2019.04.005 |
| [24] |
H. Xia, H. Li, Z. Yin, X. Hou, J.J. Zhu, ACS Appl. Mater. Interfaces 7 (2015) 696-703. DOI:10.1021/am506980d |
| [25] |
W. Yao, L. Wang, H.Y. Wang, Biosens. Bioelectron. 40 (2013) 356-361. DOI:10.1016/j.bios.2012.08.002 |
| [26] |
H.R. Zhang, Y.Z. Wang, M.S. Wu, Chem. Commun. 50 (2014) 12575-12577. DOI:10.1039/C4CC06302C |
| [27] |
X.F. Tang, D. Zhao, J.C. He, Anal. Chem. 85 (2013) 1711-1718. DOI:10.1021/ac303025y |
| [28] |
K. Shao, J. Wang, X.C. Jiang, et al., Anal. Chem. 86 (2014) 5749-5757. DOI:10.1021/ac500175y |
| [29] |
H.Y. Yang, H.J. Wang, C.Y. Xiong, et al., Electrochim. Acta 213 (2016) 512-519. DOI:10.1016/j.electacta.2016.07.149 |
| [30] |
S. Zadran, S. Standley, K. Wong, et al., Appl. Microbiol. Biot. 96 (2016) 895-902. DOI:10.1007/s00253-012-4449-6 |
| [31] |
P. Wu, X.D. Hou, J.J. Xu, H.Y. Chen, Chem. Rev. 114 (2014) 11027-11059. DOI:10.1021/cr400710z |
| [32] |
R. Wargnier, A.V. Baranov, V.G. Maslov, Nano Lett. 4 (2004) 451-457. DOI:10.1021/nl0350938 |
| [33] |
H.J. Wang, Y.Q. Chai, H. Li, R. Yuan, Biosens. Bioelectron. 100 (2018) 35-40. DOI:10.1016/j.bios.2017.08.054 |
| [34] |
G.F. Jie, J.J. Zhang, D.C. Wang, et al., Anal. Chem. 80 (2008) 4033-4039. DOI:10.1021/ac800052g |
| [35] |
L.H. Tian, L. Liu, Y.Y. Li, Q. Wei, W. Cao, Sci. Rep. 6 (2016) 1-7. DOI:10.1038/s41598-016-0001-8 |
| [36] |
K. Muzyka, Biosens. Bioelectron. 54 (2014) 393-407. DOI:10.1016/j.bios.2013.11.011 |
| [37] |
F.S. Bitaraf, I. Rasooli, S.L.M. Gargari, Eur. J. Clin. Microbiol. Infect. Dis. 35 (2016) 503-510. DOI:10.1007/s10096-015-2567-7 |
| [38] |
X.Y. Jiang, H.J. Wang, H.J. Wang, et al., Anal. Chem. 89 (2017) 4280-4286. DOI:10.1021/acs.analchem.7b00347 |
| [39] |
Y.T. Liu, H.J. Wang, C.Y. Xiong, Y.Q. Chai, R. Yuan, Biosens. Bioelectron. 87 (2017) 779-785. DOI:10.1016/j.bios.2016.08.109 |
| [40] |
T.M. Herne, M.J. Tarlov, J. Am. Chem. Soc. 119 (1997) 8916-8920. DOI:10.1021/ja9719586 |
| [41] |
Y. Chai, D. Tian, H. Cui, Anal. Chim. Acta 715 (2012) 86-92. DOI:10.1016/j.aca.2011.12.006 |
| [42] |
Y. Li, Y. Zhang, L. Jiang, Sci. Rep. 6 (2016) 22694.
|
| [43] |
M.T. Carter, A.J. Bard, Bioconjug. Chem. 1 (1990) 257-263. DOI:10.1021/bc00004a005 |
| [44] |
M. Rodriguez, A.J. Bard, Anal. Chem. 62 (1990) 2658-2662. DOI:10.1021/ac00223a002 |
| [45] |
L. Dennany, R.J. Forster, J.F. Rusling, J. Am. Chem. Soc. 125 (2003) 5213-5218. DOI:10.1021/ja0296529 |
| [46] |
L.L. Liu, X.Y. Wang, Q. Ma, Z.H. Lin, X.G. Su, Anal. Chim. Acta 916 (2016) 92-101. DOI:10.1016/j.aca.2016.02.024 |
| [47] |
L.C. Clark, C. Lyons, Ann. N. Y. Acad. Sci. 102 (1962) 29-45. |
| [48] |
S. Updike, G. Hicks, Nature 214 (1967) 986-988. DOI:10.1038/214986a0 |
| [49] |
R. Wilson, A.P. Turner, Biosens. Bioelectron. 7 (1992) 165-185. DOI:10.1016/0956-5663(92)87013-F |
| [50] |
Y. Du, X.L. Luo, J.J. Xu, Biosens. Bioelectron. 70 (2007) 342-347. DOI:10.1016/j.bioelechem.2006.05.002 |
| [51] |
F. Wu, J. Xu, Y. Tian, Biosens. Bioelectron. 24 (2008) 198-203. DOI:10.1016/j.bios.2008.03.031 |
| [52] |
X. Lu, J. Hu, X. Yao, Biomacromolecules 7 (2006) 975-980. DOI:10.1021/bm050933t |
| [53] |
T. Li, Z. Yao, L. Ding, Sens. Actuator. B-Chem. 101 (2004) 155-160. DOI:10.1016/j.snb.2004.02.047 |
| [54] |
Y.T. Kong, M. Boopathi, Y.B. Shim, Biosens. Bioelectron. 19 (2003) 227-232. DOI:10.1016/S0956-5663(03)00216-1 |
| [55] |
X. Chen, H. Ye, W. Wang, Electroanalysis 22 (2010) 2347-2352. DOI:10.1002/elan.201000095 |
| [56] |
D.P. Long, C.C. Chen, C.Y. Cui, P.H. Yang, Nanoscale 10 (2018) 18597-18605. DOI:10.1039/C8NR03847C |
| [57] |
D. Wang, Y.N. Zheng, Y.Q. Chai, Y.L. Yuan, R. Yuan, Chem. Commun. 51 (2015) 10521-10523. DOI:10.1039/C5CC02148K |
 2019, Vol. 30
2019, Vol. 30 

