Fluorescent bioanalysis, as one of the most common methods in modern analytical science, exhibits many advantages such as visualization, fast measurement, simple operation and high sensitivity, and provides key information for fundamental research, biological detection and imaging [1-3]. For example, fluorescence biosensing offers a simple and efficient tool for the detection of biomarkers [4] and toxic chemicals [5, 6], playing an important role in food safety [7, 8], environmental analysis [9], disease diagnosis [10, 11] and criminal investigation [12]. Besides, fluorescence bioimaging technologies which can visualize a series of biological processes and light specific disease landmarks, are widely applied in medical diagnosis [13-15], disease treatment [16, 17] and investigation of biological mechanisms [18, 19]. Although fluorescent bioanalysis is of the essence, it still remains a great challenge for the sensitive and specific analysis of complex samples. For practical samples such as serum and urine, there are a large number of matrix impurities which can generate strong autofluorescence and result in severe interference to the signal of targets [20]. Consequently, the signal to noise ratio of fluorescent bioanalysis decreases, leading to a decrease in the sensitivity. Therefore, it is of critical significance to eliminate the background fluorescence of impurities and improve the sensitivity for the analysis of complex samples.
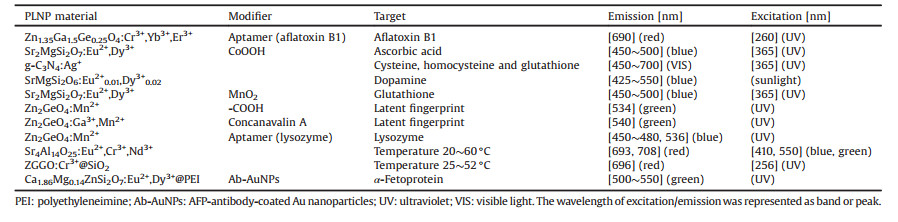
Persistent luminescence nanoparticles (PLNPs) refer to a series of luminescent nanomaterials that can swiftly store the excitation energy and emit persistently after ceasing the excitation [21-23]. Given that the lifetime of most biological fluorescence is below nanosecond level, such short-lived autofluorescence in complex samples decays fast, while the luminescence of PLNPs can maintains several seconds to hours and remains detectable after removing of the excitation [24-26] (Fig. 1). In this regard, PLNPs can effectively diminish the interference of autofluorescence and background noise, thereby extremely improving the signal-tonoise ratio and sensitivity of fluorescent analysis. Recently, the biological applications of PLNPs arouse great interest among researchers. In 2007, Chermont et al. reported their innovative work for using PLNPs in bioimaging [27], which represents that a new door was opened for bioimaging based on PLNPs. After that, PLNPs with different excitation and emission wavelength have been developed and utilized to construct luminescent nanoprobes [28-30], holding great promise for applications in bioanalysis [31].

|
Download:
|
| Fig. 1. Schematic diagram of autofluorescence elimination based on PLNPs. PLNPs can maintain several seconds to hours and remains detectable after removing of the excitation (blue). The lifetime of persistent luminescence is far longer than background fluorescence (red). | |
In this review article, we generally elaborate a summary of the recent advance in the biosensing and bioimaging applications of PLNPs. First, we briefly discuss the mechanism of persistent luminescence. Second, we introduce biosensing technology based on PLNPs, especially the protein, small molecular detection in intricate systems. Then, bioimaging applications based on PLNPs including disease diagnosis and treatment guidance are summarized. Also, we assess the current challenges of biosensing and bioimaging based on PLNPs, and put forward the development prospect of this research area. The aim of this article is to supply valuable information for the design and applications of luminescent probes, making positive influence on the development of fluorescent analysis.
2. Succinct description of the afterglow mechanismGenerally, it is of great essence to modulate the luminescent properties of PLNPs for their applications in biosensing and bioimaging. For example, PLNPs with a longer afterglow lifetime can be dramatically beneficial to the long-term tracing for biological processes [14]. In addition, extending the emission wavelength of PLNPs into the near-infrared region can also improve the tissue penetration in bioimaging [32]. Comprehending the mechanism of persistent luminescence is tremendously necessary to control suitable luminescent properties of PLNPs. In this section, we will recommend the brief luminescence mechanism of PLNPs.
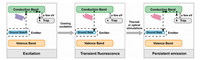
The Schematic diagram of the afterglow mechanism is shown in Fig. 2. At first, the electrons in ground state of emitter ions are excited via excitation light and transfer into conduction band of host material, and some of electrons in conduction band will be captured by electron traps [33]. After ceasing the excitation light, most of electrons in conduction band will return to ground state and cause transient fluorescence, but those in traps will still remain in electron traps. While the trapped electrons received necessary energy via thermal stimulation or optical stimulation, they will be released into conduction band and return to ground state with the energy in the form of emission.
|
Download:
|
| Fig. 2. Schematic diagram of mechanism of persistent luminescence. The electrons in ground state of emitter ions are excited via excitation light and transfer into conduction band of host materials, and some of electrons in conduction band will be captured by electron traps (left). After ceasing the excitation light, most of electrons in conduction band will return to ground state and cause transient fluorescence (middle). During thermal or optical stimulations, some electrons in electron traps are released to conduction band and return to ground state causing long lifetime emission (right). | |
During persistent luminescence process, emitters and electron traps are two cardinal elements of PLNPs [34]. The emitters usually consist of lanthanide or transition metal ions doping in host material, such as Ce3+, Cr3+, Mn2+. These ions will form several sporadic energy levels, and provide activatable electrons in the course of excitation process [21]. Electron traps are commonly formed by intrinsically crystal defects or ions co-doping in host material [35]. The energy of electron traps is often a little lower than the conduction band of host material, and electrons caught by traps return to conduction band ordinarily need thermal stimulations or optical stimulations. The number of electrons in traps determines the duration of persistent emission, and the rate of the electrons in traps return to ground state dictates to the intensity of emission [36]. Thus, choosing adaptable doping ions and controlling suitable depth of traps are of importance for PLNPs synthesis, which can influence many luminescent properties like excitation wavelength, emission wavelength and lifetime. The PLNPs for bioimaging usually need long lifetime and near-infrared emission wavelength so as to improve the imaging depth of tissue and imaging duration [16]. Except for bioimaging, the PLNPs for biosensing also need suitable excitation and emission wavelength in order to enhance the sensitive and operability of detection [37].
3. Biosensing based on PLNPsFluorescent biosensing, as one of the most important technologies for bioanalysis [38], has been widely exploited in the detection of various biological indicators (e.g. protein [39], nucleic acid [40, 41], biological small molecule [42-44], etc.) and physiological parameters (temperature [45, 46] and pH [47, 48]). This technology plays an important part in disease diagnosis [10, 11], environmental detection [9], food safety detection [7, 8] and criminal investigation [12, 49, 50]. Owing to the unique property of persistent emission, PLNPs can effectively eliminate the background fluorescence generated by matrix in intricate system [51]. In this condition, the signal-to-noise ratio can be totally enhanced with an improvement of sensitivity. Therefore, PLNPs reflect favorable advantages and exhibit great potentials in fluorescence biosensing [52]. In this section, we will introduce the recent advance of biosensing based on PLNPs in protein detection, small molecule detection, fingerprint imaging and temperature determination, and the summary of PLNPs materials that have been applied to biosensing recently is shown in Table 1.
|
|
Table 1 Summary of PLNPs applied in biosensing recently. |
3.1. Protein detection
Protein is often directly related to gene expression, providing a wealth of information for mechanisms of cellular activity, as is a kind of important markers for early diagnosis [53]. Generally, the content of some typical protein is directly or indirectly associated with many physiological activities and diseases [54]. For this reason, the assay of protein is of significance to apprehend the mechanism of physiological activities and diseases, and the technologies of detecting protein in intricate biological systemplay crucial parts in biosensing.
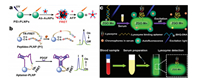
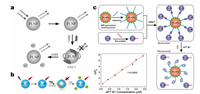
Serum alpha-fetoprotein (AFP) level is one of the important indexes to judge the physiological condition of patients in clinical medicine [55]. The increasing of AFP content levels in serum is generally under conditions of fast liver tumor cell growth and cirrhosis. Therefore, assay of AFP can provide key information in early diagnosis of hepatocellular carcinoma. In 2011, Yan and his group workers prepared Ca1.86Mg0.14ZnSi2O7:Eu2+, Dy3+ coated by polyethyleneimine (PLNPs@PEI), and conjugated with AFP-antibody-coated Au nanoparticles (Ab-AuNPs) [56]. Through fluorescence resonance energy transfer (FRET), PLNPs@PEI/Ab-AuNPs photoluminescent probe can detect AFP with high sensitivity and specificity (Fig. 3a). Because of long-lasting luminescence of PLNPs, this probe allows to detect AFP without external excitation, and effectively eliminate the autofluorescence generated by biological matrixes. In consequence, the sensitivity and accuracy of AFP detection are reallyenhanced in intricate biological surrounding. It is worth mentioning that FRET is one of the commonly used methods in fluorescence analysis. In addition to the creative work of Yan, it is also important to develop a more versatile detection platform. Ju et al. utilized PLNPs probes to design a universal timeresolved-FRET platform through setting suitable delay time and gated time for biosensing (Fig. 3b) in 2015 [57], which is further broadening the applications of PLNPs in building common platform for protein detection based on FRET. According to the authors, this platform allows to quantitatively determinate the activity of caspase-3 (associating with apoptosis) and concentration of platelet-derived growth factor (relating to cell proliferation). Based on PLNPs, the time-resolved-FRET platform effectively decreases autofluorescence of environmental medium, and shows high sensitivity, low detection limit, and a wide linear range, holding great potentials in universal protein detection platform.
|
Download:
|
| Fig. 3. (a) Schematic diagram of using PLNPs@PEI/Ab-AuNPs probe for AFP detection. Adapted with permission [56]. Copyright 2011, American Chemical Society. (b) Schematic diagram of the detection of caspase-3 (top) and platelet-derived growth factor (bottom) via time-resolved-FRET platform based on PLNPs. Adapted with permission [57]. Copyright 2015, Elsevier. (c) Schematic diagram of using Zn2GeO4:Mn2+ PLNPs for lysozyme biosensing. Adapted with permission [51]. Copyright 2017, American Chemical Society. | |
In addition to AFP, caspase-3 and platelet-derived growth factor, it is also of great significance in clinic to develop effective methods for detecting lysozyme. Lysozyme has been regarded as one of common biomarkers of clinical diagnosis, and the overexpression of lysozyme in serum is usually related to various diseases, such as AIDS [58], sarcoidosis [59], etc. The in situ detection of lysozyme in serum with high sensitivity is an important technology in clinical diagnosis. Recently, our group reported a novel method of lysozyme detection based on Zn2GeO4: Mn2+ (ZGO) persistent luminescent nanorods [51]. Specifically, ZGO were functionalized with lysozyme-binding aptamer and hybridized with BHQ-DNA (as quencher). With the existent of lysozyme, aptamers can combine with lysozyme and take off BHQDNA, which will resume luminescence of PLNPs (Fig. 3c). During the excitation of UV, the matrix of serum emitted strong autofluorescence. However, after ceasing the excitation, the autofluorescence eliminated efficiently, and the luminescence of PLNPs can last over ten minutes. In a word, this ZGO probe shows great advantages in lysozyme detection, such as great sensitivity, free autofluorescence, and high reliability. More applications of this probe in biosensing can be envisaged in the future.
3.2. Small molecule detectionSmall molecules usually play important part in the process of cell signaling and life activities. Most of time, the levels of specific molecules are often closely related to some specific disease, like Parkinson's disease, schizophrenia, Alzheimer's disease, and even cancer [60-62]. Efficient detection of these small molecules is of great significance for early diagnosis of diseases, and understanding their mechanisms. In addition to that, quantitative analysis of small molecule active substances is also an important method for food safety monitor, which can effectively provide protection of food safety and public health. In consequence, the technologies of detecting small molecules in complex biological surroundings occupy a crucial status in bioanalysis.
Biological thiols are small molecules with mercapto groups in biological metabolite, includes glutathione (GSH), homocysteine (Hcy), and cysteine (Cys). Generally, the content level of biological thiols directly or indirectly links to a series of diseases [61], such as liver damage, cancer, and even AIDS [60]. In this regard, the detection of biological thiols is of the essence [63]. In 2014, Tang et al. reported a MnO2-modified PLNPs (Sr2MgSi2O7:Eu2+, Dy3+ for detection of GSH [64]. The modification of MnO2 on the surface of the PLNPs can quench the persistent luminescence. With the existence of GSH, MnO2 will be reduced to Mn2+ ions, leading to the resume of PLNPs luminescence (Fig. 4a). Furthermore, the autofluorescence from biological samples can be effectively eliminated, in order that the detection sensitivity of GSH is totally enhanced.
|
Download:
|
| Fig. 4. (a) Schematic diagram of using MnO2-modified PLNPs (Sr2MgSi2O7:Eu2+, Dy3+) for detecting GSH. Adapted with permission [64]. Copyright 2014, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (b) Schematic diagram of using CoOOH-PLNPs probe for detection of VC via FRET. Adapted with permission [66]. Copyright 2014, American Chemical Society. (c) Schematic diagram of using CuS-PLNPs for background-free detection of AFT through FRET, and the linear response of AFT determination. Adapted with permission [37]. Copyright 2018, The Royal Society of Chemistry. | |
It is different to biological thiols that directly or indirectly relate to liver damage, cancer and AIDS, dopamine (DA) is an essential biological metabolite in nervous system, which playing an important role in neurotransmission. The abnormal level of DA content in neurons, is usually related to neurological disorders (e.g., Parkinson's disease, Alzheimer's disease, schizophrenia, etc.) [62]. Recently, Lv et al. reported their unique work in detection of DA responsive turn-off afterglow [65]. According to the authors, the persistent luminescence of PLNPs (SrMgSi2O6:Eu2+0.01, Dy3+0.02) can be effectively quenched by DA through electron transfer in the existence of oxygen. This approach shows high convenience by only mixing DA with the PLNPs, and has little interference to the dopamine detection during the existence of other phenols. Besides, it also exhibits free autofluorescence, long linear range and low detection limit of DA in intricate biological environment assay. In this situation, more applications of this probe in biosensing can be envisaged in the future.
In addition to biological metabolites, small molecules are closely associated with nutrition balance and food poisoning, including beneficial substances like trace elements, vitamin, and toxic substances like heavy metal ion and aflatoxin (AFT). Assay of these small molecules for human intake are also important. In 2014, Tang and his co-workers synthesized a highly selective and instantaneous PLNPs probe for detection of vitamin C (VC) [66]. The PLNPs was overlapped with CoOOH nanoflakes, and the luminescence of PLNPs can be quenched by CoOOH nanoflakes via FRET. In the existence of VC, CoOOH will be reduced to Co2+ ions in order to resume the luminescence of PLNPs (Fig. 4b). After ceasing the excitation, the afterglow of PLNPs can last several hours so that the autofluorescence produced in situ can be effectively eliminated. In addition to detection of VC, detection of AFTalso plays important roles in analysis of food. AFT is a common toxin molecule generated after food deterioration, and is also one of main culprits of food poisoning. It poses a great threat to the human body. Therefore, to develop effective AFT detection methods has important implications in the field of food safety. In 2018, Wang et al. synthesized an activatable hybrid PLNPs for background-free detection of AFT through FRET [37]. The PLNPs were ligated with CuS by DNA hybridization so as to construct activatable nanoprobes, and the aptamer-modified silica on the surface of PLNPs ensures a high selectivity of catching AFT. In addition, CuS nanoparticles were used to combine with aptamers and quench the luminescence. In the existence of AFT, AFT will compete with CuS to combine with ss-DNA so as to resume luminescence. As the concentration of AFT increases, the luminescence of PLNPs gradually enhances. Most importantly, due to the long-lasting afterglow of PLNPs, the PLNPs probe allows AFT assay without external excitation. Thus, the autofluorescence from the biological matrix is effectively eliminated, and the sensitivity of detection is totally increased. The detection method exhibits a linear response in the AFT concentration range of 0.1–2.4 μmol/L with a detection limit of 0.03 μmol/L (Fig. 4c).
3.3. OthersLatent fingerprints (LFPs) are invisible to the naked eye, and most common at crime scenes [67]. In criminal investigation, LFPs are usually imaged via powder method. However, the traditional powder method for imaging LFPs are usually affected by the color and the shape of underlying substrate, causing low contrast and large background interference [68]. Therefore, developing latent fingerprint imaging technology with high efficiency, high contrast, high selectivity and low background interference is the major challenge in criminal investigation. In 2017, our group develop a time-gated LFPs imaging strategy based on Zn2GeO4:Ga3+, Mn2+, which can specifically visualize LFPs via molecular recognition without background fluorescence [50]. This PLNPs were functionalized with a carboxyl group and treated with EDC/NHS (1-ethyl-3- (3-dimethylaminopropyl)-carbodiimide/N-hydroxysuccinimide) in order to label LFPs through reacting with the amino group in the LFPs. After ceasing the excitation, the LFPs images show wellresolved specific details without background fluorescence (Fig. 5a). Besides, our group recently also reported Zn2GeO4:Mn2+-COOH to image LFPs via pH-guided recognition [49]. Compared to molecular recognition, pH-guide recognition bases on electrostatic interactions but not covalent bond so that the imaging strategy is nondestructive. Besides, the dispersions can be laid in portable bottles, and LFPs can be imaged in situ by simply sprinkling the dispersions to the position of LFPs (Fig. 5b). Thus, this strategy exhibits high-resolution, nondestructive and background-free in LFP imaging, and has great potential for effective fingerprint recognition in criminal investigation.

|
Download:
|
| Fig. 5. (a) Schematic diagram of time-gated imaging of LFPs with Zn2GeO4:Ga3+, Mn2+ nanoparticles. Adapted with permission [50]. Copyright 2017, American Chemical Society. (b) Schematic diagram of pH-guided recognition imaging of LFPs with Zn2GeO4:Mn2+-COOH nanoparticles. Adapted with permission [49]. Copyright 2018, Tsinghua University Press and Springer-Verlag GmbH Germany, part of Springer Nature.) (c) Schematic diagram of temperature biosensing with ratiometric afterglow nanothermometers. Adapted with permission [46]. Copyright 2017, American Chemical Society. | |
In addition to LFPs imaging, nanothermometers have also received much attention from researchers because monitoring small changes in temperature located in physiological temperature is important for the diagnosis of disease. In 2017, Diaz-Torres et al. achieved a persistent luminescence nanothermometers with Sr4Al14O25:Eu2+, Cr3+, Nd3+ [45]. At the same time, Zhang et al. also reported a ratiometric afterglow nanothermometer based on Cr3+-doped zinc gallogermanate (ZGGO) [46]. Utilizing the difference of infrared emission peaks at different temperatures of PLNPs, these nanothermometers can effectively detect the tissue temperature in situ (Fig. 5c). Besides, the detection ranges of nanothermometers are both located in physiological temperature range, and exhibit a high sensitivity for temperature sensing. For these reasons, nanothermometers based on PLNPs have potentials to monitor small changes in temperature during diagnosis and treatment.
In a word, PLNPs are able to effectively eliminate the autofluorescence in intricate system biosensing. For this situation, PLNPs effectively enhance sensitivity and signal-to-noise ratio of biosensing, and exhibit unique advantages in fluorescence biosensing in intricate biological system. However, the strategies and methods of biosensing based on PLNPs are pending to develop, and more progresses of PLNPs in biosensing are still worth to prospect in the future.
4. Bioimaging based on PLNPsFluorescent bioimaging is a kind of visual and non-invasive methods to monitor biological activities, and has been deemed as one of the most cogent imaging strategies [69-72]. However, the orthodox fluorescent dyes, such as organic dyes, quantum dots, and metal complexes, usually have to be excited throughout the whole imaging process, while the high energy excitation light can also activate other molecules in biological surrounding, resulting of strong autofluorescence [20]. The strong autofluorescence will severely interfere with the fluorescent signal of the target, and impede the enhancement of imaging sensitivity and accuracy. As the fluorescence lifetime of proteins and small molecules in biological surrounding is less than nanoseconds, PLNPs are ideal candidates of bioimaging because the persistent luminescence of PLNPs is able to last for several minutes and even hours, which is significantly eliminating autofluorescence [27]. In this section, we will introduce the recent advance in bioimaging based on PLNPs, including the applications of bioimaging in disease diagnosis and guidance for treatment, especially in drug delivery, photothermal therapy and photodynamic therapy, and the summary of PLNPs materials that have been applied to bioimaging recently is shown in Table 2.
|
|
Table 2 Summary of PLNPs applied in bioimaging recently. |
4.1. Bioimaging for disease diagnosis
The demand for a growing array of advanced disease diagnostic technologies has led to the construction of new biomedical diagnostic strategies, which has also attracted many researchers' attention in imaging technology for disease diagnosis. As the development in recent years, the technology of bioimaging for disease diagnosis has already made some progress, and also exhibited higher diagnostic efficiency and fewer side effects [73, 74]. Imaging diagnostic strategy utilizes targeted imaging of disease markers or tissues to achieve disease diagnosis [75]. Therefore, bioimaging probe is one of the most important parts of this modern imaging medical diagnostic strategy, and has been widely utilized to identify the location of diseased tissue [76], monitor its distribution [77, 78], and evaluate treatment options [18]. Developing more reliable probes is the key point of bioimaging for disease diagnosis technology.
In 2017, Zhang et al. successfully synthesized five-nanometer Zn2SnO4:Cr3+, Eu2+ PLNPs for deep tissue bioimaging [13]. Zn2SnO4: Cr3+, Eu2+ probes were functionalized by hydroxyl groups, and modified with folic acid on the surface of the particles via the bridge of amino-group. As the Fig. 6a shows, these probes can effectively identify cancer cells, and emit excellent long afterglow NIR at approximately 800 nm. With the feature of NIR emission, the probes can penetrate 3 cm tissue without autofluorescence so that they can be used for deep-tissue imaging. In this situation, this five-nanometer PLNPs probes have great potentials to be applied in bioimaging for disease diagnosis. In addition to single-mode imaging for disease diagnosis, multi-mode imaging platform for disease diagnosis is usually more effective to diagnose diseases. Besides, multi-mode imaging is also a trend in the development of imaging technology for disease diagnosis in the future. Multimode imaging typically provides multi-angle imaging information, which can enhance the accuracy of disease diagnosis. Therefore, it is very important for development of multi-mode imaging probes. In 2018, Li et al. reported a kind of unique bioimaging probes based on ZnGa2O4:Cr3+ PLNPs for in vivo targeted imaging of orthotopic breast cancer [79]. The PLNPs were synthesized by hydrothermal method and shows high photoluminescence quantum yield. According to the authors, this afterglow probes can effectively target of breast cancer tumors and exhibit a long afterglow emission (up to 5 days). Except for these properties, these probes also have versatile functions. It can be used not only for afterglow luminescence in vivo bioimaging, but also in single photon emission computed tomography (Fig. 6b). In both methods, the PLNPs show out free autofluorescence and high signal-to-noise ratio. In consequence, the development of these ZnGa2O4:Cr3+ PLNPs probes has positive significance for targeted imaging and diagnosis of breast cancer.
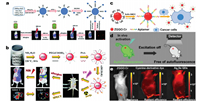
|
Download:
|
| Fig. 6. (a) Schematic illustration of the synthesis, functionalization and repeated in vivo simulated deep tissue imaging of Zn2SnO4:Cr3+, Eu2+ PLNPs. Adapted with permission [13]. Copyright 2017, The Royal Society of Chemistry. (b) Schematic illustration of the synthesis of multifunctional ZnGa2O4:Cr3+ PLNPs, and in vivo afterglow bioimaging and single photon emission computed tomography imaging. Adapted with permission [79]. Copyright 2018, The Royal Society of Chemistry. (c) Schematic diagram of aptamerfunctionalized PLNPs probes for autofluorescence-free targeted tumor imaging. (d) Schematic diagram of eliminating autofluorescence interference by PLNPs. (e) The photographs of imaging in vivo with PLNPs, cyanine-derivative dye and Ag2Se. (c–e) Adapted with permission [80]. Copyright 2017, American Chemical Society. | |
Recently, our group also developed unique PLNPs probes for autofluorescence-free targeted tumor imaging and the probes can be activated in vivo by commercial orange LED [80]. We synthesized a kind of aptamer-functionalized PLNPs, and are able to achieve cancer cell targeting bioimaging (Fig. 6c). Compared to cyaninederivative dye and Ag2Se, imaging with PLNPs can completely reduce the autofluorescence generated by biological matrix (Figs. 6d and e). The nanoprobes show high affinity for tumors and nice biocompatibility, and can also be effectively activated in vivo by commercial orange LED (550 nm). Under these conditions, these PLNPs probes are ideal materials for non-fluorescent tumortargeted bioimaging and tumor diagnosis in vivo.
4.2. Bioimaging for guiding treatmentBioimaging-guided therapy is the forefront of current biomedical therapies, especially for cancer treatment. Integrating bioimaging and therapy into a single nanoplatform can achieve higher therapeutic efficiencies, as well as lower collateral damage. Combining the imaging platform with the drug carriers can effectively monitor the distribution of the drugs in the living body to evaluate the therapeutic effect [81]. The combination of the imaging particles with the photothermal agent or the photosensitizer can directly indicate the position of the lesion irradiated by the light source of treatment, achieve efficient photothermal therapy or photodynamic therapy, and minimize the additional damage of the normal cells causing by high energy light [16, 82]. PLNPs have longlasting emission after stopping the excitation of the light source, which can effectively eliminate the background fluorescence generated by biological matrix during bioimaging. Except for that, the emission wavelength of PLNPs can be regulated to biologically transparent window in order to make high tissue penetration [83, 84]. Thus, the application of PLNPs to bioimaging-guided treatment can improve the high signal-to-noise ratio of imaging and provide guarantee for precise treatment.
4.2.1. Bioimaging-guided drug deliveryDrug carrier is a type of nano-platform loading medicine molecules through adsorption or encapsulation, and releasing the molecules accurately after delivering to the affected area, such as mesoporous silica [85, 86], liposomes [87]. The emergence of drug carriers can be used for the delivery of some poorly soluble drugs and high side effects drugs to decrease the additional damage, and improve the efficacy of drugs [88]. Bioimaging-guided drug carrier can be exploited to investigate the distribution of drug carriers in vivo through bioimaging, andcan timely evaluate the diagnosis and treatment of diseases [89]. Therefore, the development of bioimaging-guided drug carriers has received widespread attention from researchers in recent years.
In 2017, Yan et al. reported using liposome-coated PLNPs as fluorescent labeled drug carriers achieved traceable drug delivery [90]. Paclitaxel is a broad-spectrum anti-tumor drug that is waterinsoluble and difficult to modify. The authors constructed liposomes labeled with PLNPs, and encapsulated paclitaxel into liposomes, which can passively target human breast cancer xenograft in nude mice. Using the characteristics of persistent luminescence of PLNPs, the distribution of liposomes encapsulating the drug can still be monitored without autofluorescence in real time after the excitation light ceasing, and the effectiveness of medicine treatment can be readily evaluated (Fig. 7a). In addition, the drug carriers also have the advantages of high drug-loading efficiency, biocompatibility, biodegradability and low toxicity, as represented these liposome-coated PLNPs are the effective drug carriers for trackable cancer treatment.
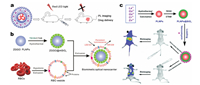
|
Download:
|
| Fig. 7. (a) Schematic diagram of time-gated imaging of liposome-coated PLNPs as fluorescent labeled drug carriers for traceable drug delivery. Adapted with permission [90]. Copyright 2017, American Chemical Society. (b) Schematic diagram of the erythrocyte membrane bioinspired PLNPs nano-carriers for in vivo bioimaging and drug delivery. Adapted with permission [91]. Copyright 2018, Elsevier. (c) Schematic diagram of mesoporous PLNPs utilizing in bioimaging-guided in vivo drug delivery to the gut. Adapted with permission [92]. Copyright 2018, The Royal Society of Chemistry. | |
In addition to liposome-coated drug carriers, drug carriers camouflaged with biofilms generally have better biocompatibility. Wang et al. presented a novel drug carrier, camouflaged with erythrocyte membrane, labeled by PLNPs and using mesoporous silica as a drug carrier for loading surface [91]. As the luminescent center of the nano-drug carriers, the near-infrared PLNPs can continue to emit light after ceasing the excitation, and the nearinfrared emission wavelength is located in biological transparent windows so that it gets high tissue penetration and can effectively monitor the distribution of the drug carrier in deep tissues. In this case, the effect of drug treatment can be readily monitored and evaluated. In addition, the mesoporous silica structure coated on the PLNP as the drug loader can improve the loading efficiency of the drug. Integrating erythrocyte membrane vesicles with PLNPs can also ensures that the developed nano-drug carriers bypass macrophage uptake and systemic clearance in order that the nanomedicine carrier has good biocompatibility (Fig. 7b). In addition, it is also very important in disease treatment to develop drug deliveries that can be not only injected but also taken orally. Wang et al. recently synthesized extremely long afterglow mesoporous PLNPs (La3Ga5GeO14:Cr3+, Zn2+) applied in bioimaging-guided in vivo drug delivery to the intestine [92]. According to theauthors, the drug delivery can be injected into the body by oralor intravenous injection. Besides, the PLNPs drug carrier can not only be excited by the UV lamp, but also can be excited or re-excited by red LED so that the distribution of carriers in vivo can be monitored for a long time without the background fluorescence generated by biological matrix. Besides, mesoporous silica is easily entangled around the core of PLNPs and is used to adsorb vancomycin(Fig. 7c). Thus, the drug carrier can be targeted to deliver vancomycin to the gut to inhibit the growth of staphylococcus aureus. In the future, PLNPs-based drug carrier is expected to develop as advance nanodrug delivery platform.
4.2.2. Bioimaging-guided photothermal therapyNIR laser-induced photothermal therapy is a non-invasive treatment strategy. Through stimulating via NIR laser, photothermal agent will generate heat at the tumor site, causing the death of cancer cells [93]. Compared with traditional therapies, photothermal therapy as a minimally invasive even non-invasive treatment, and with strong tissue penetration, has received great interest in cancer treatments [94, 95]. However, conventional photothermal therapy usually requires a wide range of NIR laser irritation, leading to strong tissue thermal damage and being difficult to achieve the desired therapeutic efficiency [96]. Bioimaging-guided photothermal therapy provides targeted imaging of tumor cell locations through imaging platforms, providing accurate targets for NIR laser irritation [82]. In this case, bioimaging-guided photothermal therapy can effectively improve the therapeutic efficiency of photothermal therapy and reduce the additional damage caused by laser irradiation.
In 2016, Yan et al. synthesized a kind of multifunctional PLNPs/CuS nanoprobe for in vivo bioimaging-guided photothermal therapy [96]. This nanoprobe was constructed by linking PLNPs and CuS via matrix metalloproteinases (MMPs) and specific peptide substrates (H2N-GPLGVRGC-SH). The modification of CuS on the surface of PLNPs can effectively quench the luminescence, but the MMPs in the tumor region can specifically cleaves the ligated peptide to resume the luminescence of PLNPs (Fig. 8a), resulting in sensitive optical imaging with high specificity and signal to noise ratio. Besides, the authors demonstrated that the nanoprobe can be used for luminescence bioimaging-guided photothermal therapy targeting cancer cell (Fig. 8b), and its multi-function of high sensitivity tumor-targeted imaging and effective photothermal treatment of tumor make the nanoprobe promise for a wider range of therapeutic applications. In addition to Yan's unique work, Chang and his co-workers also reported an accurate tracker in vivo for near-infrared photothermal therapy based on PLNPs recently [97]. The authors designed and prepared mesoporous silica nanocarriers copolymerized with indocyanine green (ICG) and PLNPs for long-lasting luminescence-guided photothermal therapy. After injecting these nanoparticles into the tumor of a living mouse, the tumor region can be accurately irradiated with 808 nm NIR light. ICG absorbs NIR and efficiently converts it into a photothermal response quickly, so as to achieve accurate and efficient killing of a large number of tumor cells (Fig. 8c). Since PLNPs have the property of long-lasting emission after ceasing the excitation, bioimaging based on PLNPs will not cause autofluorescence in situ. In consequence, this new PLNPs has great potential as a tracker for precise treatment of various tumors.
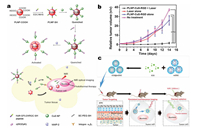
|
Download:
|
| Fig. 8. (a) Schematic diagram of PLNPs/CuS nanoprobe for in vivo bioimaging-guided photothermal therapy. (b) Tumor growth curves in each mice group after different treatments. Tumor volumes were normalized to the initial size. (a and b) Adapted with permission [96]. Copyright 2016, American Chemical Society. (c) Schematic diagram of the PLNPs@ICG-mSiO2 nanoparticles for persistent luminescent imaging-guided photothermal therapy in vivo. Adapted with permission [97]. Copyright 2017, Elsevier. | |
4.2.3. Bioimaging-guided photodynamic therapy
Except for photothermal therapy, photodynamic therapy is also a novel cancer treatment strategy [98-100]. Photodynamic therapy utilizes a photoreactive molecule called photosensitizer [101, 102]. When exposed to light of the appropriate wavelength, photosensitizer will produce singlet oxygen [103], and singlet oxygen can kill cancer cells directly, destroy tumor-associated microvasculature or stimulate anti-tumor immune in order to inhibit the growth of tumors. As the unique advantages and low systemic toxicity of photodynamic therapy, in recent years, it has received extensive attention from researchers, and has also made some progresses [16, 17]. However, a wide range of excitation light exposure during photodynamic therapy usually causes photoallergic, cellular damage, and even genetic mutations. Thus, improving the accuracy of photodynamic therapy for excitation light irradiation can effectively avoid photoallergic and achieve higher therapeutic efficiency. Bioimaging-guided photodynamic therapy can accurately lock at the location of the tumor through the imaging nano-platform and guide the precise irritation of the excitation light [104]. In this condition, the accuracy and efficiency of photodynamic therapy are totally enhanced, and the photoallergic due to the excitation light can be obviously reduced.
In 2017, Shen et al. reported a strategy of photodynamic therapy through bioimaging-guided based on PLNPs [104]. According to the authors, the nanosensitizer was combined by co-loading 2, 3- naphthalocyanine (NC) and LiGa5O8:Cr3+ to mesoporous silica nanoparticles (NC- LiGa5O8:Cr3+@mSiO2). Among these, NC can efficiently produce singlet oxygen under X-ray irradiation and kill cancer cells, and PLNPs can persistently emit after ceasing the excitation, which provides a high signal-to-noise ratio for imaging. With the autofluorescence-free imaging in vivo, operators can use X-rays to accurately irradiate to tumor cells. In this case, this strategy effectively improves the efficiency of singlet oxygen generation, and makes photodynamic therapy more efficient and more selective. At the same time, it also minimizes the additional tissue damage caused by X-ray irradiation. This progress is of great value in the development of the highly selective photodynamic therapy as a new type of cancer treatment. However, the research on imaging-guided photodynamic therapy based on PLNPs is still in its infancy. In consequence, there is an urgent need to develop more types of PLNPs which is suitable for imaging-guided photodynamic therapy.
In a word, with the afterglow property of PLNPs, bioimaging platforms based on PLNPs can significantly eliminate autofluorescence, improve imaging signal-to-noise ratio, and allow for longterm in vivo imaging. Moreover, the emission wavelength of PLNPs can be regulated into biologically transparent window, which makes PLNPs have obvious advantages of deep tissue penetration. Therefore, PLNPs have been widely applied in disease diagnosis, imaging-guided drug delivery, photothermal therapy and photodynamic therapy, and all of them have good development prospects in the future.
5. Conclusion and outlookOwing to its afterglow luminescence after ceasing the excitation, PLNPs have already received widespread interest in analytical science by researchers. In this review, we discuss the advances of PLNPs in biosensing and bioimaging. As in situ excitation is not necessary, PLNPs have unique advantages in eliminating autofluorescence interference and are ideal materials for biosensing in intricate biological environments. The bioimaging based on PLNPs plays significant roles in disease detection, food safety, and criminal investigation. In recent decades, researchers designed a series of reasonable PLNPs which have improved the continuous luminescence intensity, extended the decay time, and regulated the emission wavelength in the biological transparent window. In this condition, PLNPs can be well applied in long-term, deep tissue and autofluorescence-free bioimaging. In addition, bioimaging-guided treatment strategies based on PLNPs can provide high signal-to-noise ratio visualization cancer treatments. PLNPs can effectively monitor the distribution of drug carriers in vivo and evaluate the therapeutic effect in real time. Although great progress has been made, the applications of PLNPs in biosensing and bioimaging are still in infancy. At the present stage, PLNPs used for biosensing still face to several problems, such as poor dispersion and easy agglomeration. At the same time, it is also important to develop more universal sensing platforms based on PLNPs, especially based on the PLNPs excited by low-energy excitation light. Thus, more advanced strategies and methods of biosensing based on PLNPs are pending to develop, and more progresses of PLNPs in biosensing are still worth to prospect in the future. In addition, for PLNPs applied in bioimaging, there are still several problems waiting for solving: (1) PLNPs are usually doped with heavy metal ions such as rare earth ions, which inevitably cause some damage to the organism. (2) Most of the traditional PLNPs need to be excited by ultraviolet light. This high-energy excitation light will cause a certain degree of damage to the organism, and has a low tissue penetration ability. It is necessary to develop more types of PLNPs suitable for long-time and deep-tissue bioimaging, especially more universal imaging platforms based on PLNPs. In short, PLNPs have shown unprecedented advantages in biosensing and bioimaging, and will play more important parts in development of bioanalytical science in the future.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21675120), the National Key R&D Program of China (Nos. 2017YFA0208000, 2016YFF0100800), the National Postdoctoral Program for Innovative Talents (No. BX20180223), the National Basic Research Program of China (973 Program, No. 2015CB932600) and the Ten Thousand Talents Program for Young Talents. Q. Yuan thanks the Large-scale Instrument and Equipment Sharing Foundation of Wuhan University.
| [1] |
S. Kunjachan, F. Gremse, B. Theek, et al., ACS Nano 7 (2013) 252-262. DOI:10.1021/nn303955n |
| [2] |
H. Liu, Y. Sun, Z. Li, et al., Nanoscale 11 (2019) 8458-8463. DOI:10.1039/C9NR01678C |
| [3] |
J. Yao, M. Yang, Y. Duan, Chem. Rev. 114 (2014) 6130-6178. DOI:10.1021/cr200359p |
| [4] |
N. Ma, W. Jiang, T. Li, et al., Microchim. Acta 182 (2015) 443-447. DOI:10.1007/s00604-014-1386-3 |
| [5] |
Y. Li, X. Xie, Xe Yang, et al., Chem. Sci. 8 (2017) 4006-4011. DOI:10.1039/C7SC00303J |
| [6] |
D. Tian, X.J. Liu, R. Feng, et al., ACS Appl. Mater. Interfaces 10 (2018) 5618-5625. DOI:10.1021/acsami.7b15764 |
| [7] |
X. Wang, T. Hou, S. Dong, X. Liu, F. Li, Biosens. Bioelectron. 77 (2016) 644-649. DOI:10.1016/j.bios.2015.10.034 |
| [8] |
L. Xu, Z. Lu, L. Cao, et al., Food Control 75 (2017) 21-28. DOI:10.1016/j.foodcont.2016.12.018 |
| [9] |
X. Zuo, H. Zhang, Q. Zhu, et al., Biosens. Bioelectron. 85 (2016) 464-470. DOI:10.1016/j.bios.2016.05.044 |
| [10] |
S.C. Lee, H.H. Park, S.H. Kim, et al., Anal. Chem. 91 (2019) 5573-5581. DOI:10.1021/acs.analchem.8b03735 |
| [11] |
X. Qian, Z. Xu, Chem. Soc. Rev. 44 (2015) 4487-4493. DOI:10.1039/C4CS00292J |
| [12] |
J. Wang, T. Wei, X. Li, et al., Angew. Chem. Int. Ed. 53 (2014) 1616-1620. DOI:10.1002/anie.201308843 |
| [13] |
J.L. Li, J.P. Shi, C.C. Wang, et al., Nanoscale 9 (2017) 8631-8638. DOI:10.1039/C7NR02468A |
| [14] |
X.H. Lin, L. Song, S. Chen, et al., ACSAppl.Mater. Interfaces 9 (2017) 41181-41187. DOI:10.1021/acsami.7b13920 |
| [15] |
E. Teston, T. Maldiney, I. Marangon, et al., Small 14 (2018) e1800020. DOI:10.1002/smll.201800020 |
| [16] |
R. Abdurahman, C.X. Yang, X.P. Yan, Chem. Commun. 52 (2016) 13303-13306. DOI:10.1039/C6CC07616E |
| [17] |
L. Hu, P. Wang, M. Zhao, et al., Biomaterials 163 (2018) 154-162. DOI:10.1016/j.biomaterials.2018.02.029 |
| [18] |
N. Yu, L. Huang, Y. Zhou, et al., Adv. Healthcare Mater. 8 (2019) e1801132. DOI:10.1002/adhm.201801132 |
| [19] |
S. Chen, A.Z. Weitemier, X. Zeng, et al., Science 359 (2018) 679-684. DOI:10.1126/science.aaq1144 |
| [20] |
J.M. Baumes, J.J. Gassensmith, J. Giblin, et al., Nat. Chem. 2 (2010) 1025-1030. DOI:10.1038/nchem.871 |
| [21] |
Y. Du, Y. Jiang, T. Sun, et al., Adv. Mater. 31 (2018) e1807062. |
| [22] |
Y. Xia, H. Ou, W. Li, G. Han, Z. Li, Nanomaterials 8 (2018) 260. DOI:10.3390/nano8040260 |
| [23] |
Z. Pan, Y.Y. Lu, F. Liu, Nat. Mater. 11 (2011) 58-63. |
| [24] |
H. Liu, X. Hu, J. Wang, et al., Chin. Chem. Lett. 29 (2018) 1641-1644. DOI:10.1016/j.cclet.2018.02.005 |
| [25] |
P.J. Liu, Y.L. Liu, Chin. Chem. Lett. 11 (2000) 843-846. |
| [26] |
N. Yu, Y. Li, Z. Li, G. Han, Sci. China Chem. 61 (2018) 757-758. DOI:10.1007/s11426-018-9263-9 |
| [27] |
Q.M. de Chermont, C. Chanéac, J. Seguin, et al., Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 9266-9271. DOI:10.1073/pnas.0702427104 |
| [28] |
Z. Li, L. Huang, Y. Zhang, et al., Nano Res. 10 (2017) 1840-1846. DOI:10.1007/s12274-017-1548-9 |
| [29] |
R. Shrivastava, J. Kaur, Chin. Chem. Lett. 26 (2015) 1187-1190. DOI:10.1016/j.cclet.2015.05.028 |
| [30] |
Y. Xia, X. Huang, W. Wu, et al., J. Lumin. 207 (2019) 53-57. DOI:10.1016/j.jlumin.2018.11.005 |
| [31] |
Z. Yu, B. Liu, W. Pan, et al., Chem. Commun. 54 (2018) 3504-3507. DOI:10.1039/C8CC00743H |
| [32] |
Z. Xue, X. Li, Y. Li, et al., Nanoscale 9 (2017) 7276-7283. DOI:10.1039/C6NR09716B |
| [33] |
Y. Li, S. Zhou, G. Dong, et al., Sci. Rep. 4 (2014) 4059. |
| [34] |
B. Qu, B. Zhang, L. Wang, R. Zhou, X.C. Zeng, Chem. Mater. 27 (2015) 2195-2202. DOI:10.1021/acs.chemmater.5b00288 |
| [35] |
F. Wang, B. Yang, D. Liu, et al., Spectrochim. Acta A Mol. Biomol. Spectrosc. 126 (2014) 46-52. DOI:10.1016/j.saa.2014.01.102 |
| [36] |
Y. Li, M. Gecevicius, J. Qiu, Chem. Soc. Rev. 45 (2016) 2090-2136. DOI:10.1039/C5CS00582E |
| [37] |
J.M. Liu, X.Y. Yuan, H.L. Liu, D. Cheng, S. Wang, RSC Adv. 8 (2018) 28414-28420. DOI:10.1039/C8RA05555F |
| [38] |
J. Chen, Q. Chen, C. Gao, et al., J. Mater. Chem. B 3 (2015) 964-967. DOI:10.1039/C4TB01875C |
| [39] |
P. Zou, Y. Liu, H. Wang, et al., Biosens. Bioelectron. 79 (2016) 29-33. DOI:10.1016/j.bios.2015.12.012 |
| [40] |
W. Pan, B. Liu, X. Gao, et al., Nanoscale 10 (2018) 14264-14271. DOI:10.1039/C8NR04106G |
| [41] |
J. Chen, H. Qiu, M. Zhang, et al., Biosens. Bioelectron. 68 (2015) 550-555. DOI:10.1016/j.bios.2015.01.054 |
| [42] |
F. Qu, Y. Liu, R. Kong, J. You, Microchim. Acta 184 (2017) 4417-4424. DOI:10.1007/s00604-017-2486-7 |
| [43] |
M. Hoang, P.J.J. Huang, J. Liu, ACS Sens. 1 (2016) 137-143. DOI:10.1021/acssensors.5b00147 |
| [44] |
X. Wang, B. Liu, J. Liu, Langmuir 34 (2018) 15871-15877. DOI:10.1021/acs.langmuir.8b03335 |
| [45] |
E.M. Rodríguez, G. López-Peña, E. Montes, et al., Appl. Phys. Lett. 111 (2017) 081901. DOI:10.1063/1.4990873 |
| [46] |
J. Yang, Y. Liu, Y. Zhao, et al., Chem. Mater. 29 (2017) 8119-8131. DOI:10.1021/acs.chemmater.7b01958 |
| [47] |
Y. Han, C. Ding, J. Zhou, Y. Tian, Anal. Chem. 87 (2015) 5333-5339. DOI:10.1021/acs.analchem.5b00628 |
| [48] |
H. Huang, F. Dong, Y. Tian, Anal. Chem. 88 (2016) 12294-12302. DOI:10.1021/acs.analchem.6b03470 |
| [49] |
Z.H. Li, Q. Wang, Q. Wang, et al., Nano Res. 11 (2018) 6167-6176. DOI:10.1007/s12274-018-2133-6 |
| [50] |
J. Wang, Q. Ma, H. Liu, et al., Anal. Chem. 89 (2017) 12764-12770. DOI:10.1021/acs.analchem.7b03003 |
| [51] |
J. Wang, Q. Ma, W. Zheng, et al., ACS Nano 11 (2017) 8185-8191. DOI:10.1021/acsnano.7b03128 |
| [52] |
S.K. Sun, H.F. Wang, X.P. Yan, Acc. Chem. Res. 51 (2018) 1131-1143. DOI:10.1021/acs.accounts.7b00619 |
| [53] |
J.D. Lapek Jr, P. Greninger, R. Morris, et al., Nat. Biotechnol. 35 (2017) 983-989. DOI:10.1038/nbt.3955 |
| [54] |
J.D. Cohen, A.A. Javed, C. Thoburn, et al., Proc. Natl. Acad. Sci. U. S. A. 114 (2017) 10202-10207. DOI:10.1073/pnas.1704961114 |
| [55] |
Y. Lu, M. Zhu, W. Li, et al., J. Cell. Mol. Med. 20 (2016) 549-558. DOI:10.1111/jcmm.12745 |
| [56] |
B.Y. Wu, H.F. Wang, J.T. Chen, X.P. Yan, J. Am. Chem. Soc. 133 (2011) 686-688. DOI:10.1021/ja108788p |
| [57] |
L. Zhang, J. Lei, J. Liu, F. Ma, H. Ju, Biomaterials 67 (2015) 323-334. DOI:10.1016/j.biomaterials.2015.07.037 |
| [58] |
M.H. Grieco, M.M. Reddy, H.B. Kothari, et al., Clin. Immunol. Immnopathol. 32 (1984) 174-184. DOI:10.1016/0090-1229(84)90119-3 |
| [59] |
R.S. Pascual, J.B.L. Gee, S.C. Finch, N. Engl. J. Med. 289 (1973) 1074-1076. DOI:10.1056/NEJM197311152892007 |
| [60] |
L.A. Herzenberg, S.C. de Rosa, J.G. Dubs, et al., Proc. Natl. Acad. Sci. U. S. A. 94 (1997) 1967-1972. DOI:10.1073/pnas.94.5.1967 |
| [61] |
D.M. Townsend, K.D. Tew, H. Tapiero, Biomed. Pharmacother. 57 (2003) 145-155. DOI:10.1016/S0753-3322(03)00043-X |
| [62] |
A. Gupta, Y. Wang, H. Markram, Science 287 (2000) 273-278. DOI:10.1126/science.287.5451.273 |
| [63] |
Y. Tang, H. Song, Y. Su, Y. Lv, Anal. Chem. 85 (2013) 11876-11884. DOI:10.1021/ac403517u |
| [64] |
N. Li, W. Diao, Y. Han, et al., Chem.-Eur. J. 20 (2014) 16488-16491. DOI:10.1002/chem.201404625 |
| [65] |
J. Tang, Y. Su, D. Deng, et al., Analyst 141 (2016) 5366-5373. DOI:10.1039/C6AN00882H |
| [66] |
N. Li, Y. Li, Y. Han, et al., Anal. Chem. 86 (2014) 3924-3930. DOI:10.1021/ac5000587 |
| [67] |
M. Wang, M. Li, A. Yu, et al., Adv. Funct. Mater. 27 (2017) 1606243. DOI:10.1002/adfm.201606243 |
| [68] |
M. Wang, M. Li, A. Yu, J. Wu, C. Mao, ACS Appl. Mater. Interfaces 7 (2015) 28110-28115. DOI:10.1021/acsami.5b09320 |
| [69] |
Q. Zhao, C. Huang, F. Li, Chem. Soc. Rev. 40 (2011) 2508-2524. DOI:10.1039/c0cs00114g |
| [70] |
Q. Zhao, M. Yu, L. Shi, et al., Organometallics 29 (2010) 1085-1091. DOI:10.1021/om900691r |
| [71] |
J. Xu, L. Shang, Chin. Chem. Lett. 29 (2018) 1436-1444. DOI:10.1016/j.cclet.2017.12.020 |
| [72] |
Z. Xu, J. Chen, L.L. Hu, et al., Chin. Chem. Lett. 28 (2017) 1935-1942. DOI:10.1016/j.cclet.2017.07.018 |
| [73] |
Y.C. Lu, C.X. Yang, X.P. Yan, Nanoscale 7 (2015) 17929-17937. DOI:10.1039/C5NR05623C |
| [74] |
J. Shi, X. Sun, J. Zhu, J. Li, H. Zhang, Nanoscale 8 (2016) 9798-9804. DOI:10.1039/C6NR00590J |
| [75] |
Y. Yang, Q. Zhao, W. Feng, F. Li, Chem. Rev. 113 (2013) 192-270. DOI:10.1021/cr2004103 |
| [76] |
L. Song, X.H. Lin, X.R. Song, et al., Nanoscale 9 (2017) 2718-2722. DOI:10.1039/C6NR09553D |
| [77] |
Z. Zhou, W. Zheng, J. Kong, et al., Nanoscale 9 (2017) 6846-6853. DOI:10.1039/C7NR01209H |
| [78] |
W. Zheng, P. Huang, D. Tu, et al., Chem. Soc. Rev. 44 (2015) 1379-1415. DOI:10.1039/C4CS00178H |
| [79] |
H. Liu, F. Ren, H. Zhang, et al., J. Mater. Chem. B 6 (2018) 1508-1518. DOI:10.1039/C7TB03148C |
| [80] |
J. Wang, Q. Ma, X.X. Hu, et al., ACS Nano 11 (2017) 8010-8017. DOI:10.1021/acsnano.7b02643 |
| [81] |
T. Maldiney, B. Ballet, M. Bessodes, D. Scherman, C. Richard, Nanoscale 6 (2014) 13970-13976. DOI:10.1039/C4NR03843F |
| [82] |
B. Zheng, H.B. Chen, P.Q. Zhao, et al., ACS Appl. Mater. Interfaces 8 (2016) 21603-21611. DOI:10.1021/acsami.6b07642 |
| [83] |
Y. Li, S. Zhou, Y. Li, et al., J. Mater. Chem. C 2 (2014) 2657-2663. |
| [84] |
R. Zou, J. Huang, J. Shi, et al., Nano Res. 10 (2017) 2070-2082. DOI:10.1007/s12274-016-1396-z |
| [85] |
D. Tarn, C.E. Ashley, M. Xue, et al., Acc. Chem. Res. 46 (2013) 792-801. DOI:10.1021/ar3000986 |
| [86] |
S.H. Wu, Y. Hung, C.Y. Mou, Chem. Commun. 47 (2011) 9972-9985. DOI:10.1039/c1cc11760b |
| [87] |
B. Ozpolat, A.K. Sood, G. Lopez-Berestein, Adv. Drug Deliv. Rev. 66 (2014) 110-116. DOI:10.1016/j.addr.2013.12.008 |
| [88] |
S. Mura, J. Nicolas, P. Couvreur, Nat. Mater. 12 (2013) 991-1003. DOI:10.1038/nmat3776 |
| [89] |
M. Luan, J. Chang, W. Pan, et al., Anal. Chem. 90 (2018) 10951-10957. DOI:10.1021/acs.analchem.8b02494 |
| [90] |
L.J. Chen, C.X. Yang, X.P. Yan, Anal. Chem. 89 (2017) 6936-6939. DOI:10.1021/acs.analchem.7b01397 |
| [91] |
J.M. Liu, D.D. Zhang, G.Z. Fang, S. Wang, Biomaterials 165 (2018) 39-47. DOI:10.1016/j.biomaterials.2018.02.042 |
| [92] |
D.D. Zhang, J.M. Liu, N. Song, et al., J. Mater. Chem. B 6 (2018) 1479-1488. DOI:10.1039/C7TB02759A |
| [93] |
H. Wang, J. Chang, M. Shi, et al., Angew. Chem. Int. Ed. 58 (2019) 1057-1061. DOI:10.1002/anie.201811273 |
| [94] |
X. Huang, I.H. El-Sayed, W. Qian, M.A. El-Sayed, J. Am. Chem. Soc. 128 (2006) 2115-2120. DOI:10.1021/ja057254a |
| [95] |
J.T. Robinson, S.M. Tabakman, Y. Liang, et al., J. Am. Chem. Soc. 133 (2011) 6825-6831. DOI:10.1021/ja2010175 |
| [96] |
L.J. Chen, S.K. Sun, Y. Wang, et al., ACS Appl. Mater. Interfaces 8 (2016) 32667-32674. DOI:10.1021/acsami.6b10702 |
| [97] |
H. Chen, B. Zheng, C. Liang, et al., Mater. Sci. Eng. C 79 (2017) 372-381. DOI:10.1016/j.msec.2017.05.053 |
| [98] |
H.D. Cui, D.H. Hu, J.N. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1391-1398. DOI:10.1016/j.cclet.2016.12.038 |
| [99] |
L. Song, P.P. Li, W. Yang, et al., Adv. Funct. Mater. 28 (2018) 1707496. DOI:10.1002/adfm.201707496 |
| [100] |
Y. Zhu, W. Lin, W. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1875-1877. DOI:10.1016/j.cclet.2017.06.017 |
| [101] |
L.L. Rui, H.L. Cao, Y.D. Xue, et al., Chin. Chem. Lett. 27 (2016) 1412-1420. DOI:10.1016/j.cclet.2016.07.011 |
| [102] |
Z. Yu, P. Zhou, W. Pan, N. Li, B. Tang, Nat. Commun. 9 (2018) 5044. DOI:10.1038/s41467-018-07197-8 |
| [103] |
Q. Chen, Y. Ma, J. Zhao, et al., Chin. Chem. Lett. 29 (2018) 1171-1178. DOI:10.1016/j.cclet.2018.04.025 |
| [104] |
H. Chen, X. Sun, G.D. Wang, et al., Mater. Horiz. 4 (2017) 1092-1101. DOI:10.1039/C7MH00442G |
 2019, Vol. 30
2019, Vol. 30