b Tianjin University of Traditional Chinese Medicine, Tianjin 300193, China
Aromatic β-carboline derivatives are frequently found in naturally occurring substances and synthetic analogues [1]. They have proven to be useful in the treatment of malaria [2], cancer [3], AIDS [4], and other diseases [5]. They also act as photosensitizers in material science [6]. In view of the importance of the β-carboline motif, particularly in medicinal chemistry, the green and practical methods for the preparation of these compounds are desirable. In the traditional methods such as Pictet-Spengler reaction [7] and Bischler-Napieralski cyclization [8], a protic or Lewis acid is needed to obtain the imine intermediate and the reactions take place under harsh conditions. Moreover, a subsequent oxidation step is required to form the desired aromatic β-carbolines [9]. Recently, oxidative cycloaromatization reactions for the synthesis of β- carbolines have been developed by use of transition-metal catalysts including Pd [10], Cu [11], Ru [12], Rh [13], Au [14] and bimetallic Cu/Rh [15]. Although these reported protocols were successfully applied to syntheses of β-carboline derivatives, most of the reactions occurred in the presence of expensive metal catalysts, and in certain cases the starting materials were not easily accessible. In 2013, Wu and co-workers developed a metal-free cascade cycloaromatization reaction of aromatic ketones with tryptamines through the use of a combination of I2 and H2O2. Diverse natural products containing a core structure of β-carboline were efficiently constructed in one-step under their reaction conditions [16]. During the last decade the readily available iodine reagents such as molecular iodine and iodide salts have been studied as environmentally friendly alternatives to metal catalysts in the oxidative C-H bond functionalization [17]. In contrast, the bromine-catalytic version of the oxidative C-H bond functionalization was mainly unexplored until 2013 when Glorius and Li independently reported tetraalkylammonium bromide catalyzed intramolecular dehydrogenative arylation of aldehydes that allows straightforward access to fluorenones and xanthones [18, 19]. More recently, some novel transformations were achieved by use of bromine catalysts rather than iodine catalysts [20]. As part of our continuous efforts in searching for the metal-free halide (iodine and bromine)-mediated cross-dehydrogenative coupling (HCDC) reactions [20, 21], we report herein an nBu4NBr-mediated cycloaromatization of tryptophan derivatives with aldehydes to prepare β-carbolines in one-step. This approach is characterized by its readily available starting materials, mild conditions, operational simplicity and multigram scale synthesis while relying on nBu4NBr as an environmentally benign and cheap mediator.
At the outset of our investigation, the readily available tryptophan methyl ester hydrochloride 1a and benzaldehyde 2a were employed as the model substrates, and various catalysts were examined using hydroperoxides as oxidants (Table 1). When I2 was used as catalyst, trace amounts of product 3aa was detected and starting material was reclaimed (entry 1). We previously reported that the combination of I2/TBHP promoted an intramolecular annulation of 1a to afford pyrrolo[2, 3-b]indole 4 using 1, 4-dioxane as the solvent (Table 1) [21a]. Interestingly, no intramolecular annulation product was observed under the present reaction conditions. The reaction was then investigated with nBu4NI/TBHP system, and the desired product 3aa was obtained in 52% yield (entry 2). When nBu4NBr was used instead of nBu4NI, a higher yield of 3aa was observed (entry 3). We further found that the combination of nBu4NBr with cumene hydroperoxide (CHP) promoted the cycloaromatization more efficiently to provide β-carboline 3aa in 91% yield (entry 4). In 2011, Wan and coworkers reported an nBu4NI-catalyzed cross-dehydrogenative coupling reaction of aldehyde with TBHP to prepare tert-butyl perester 5 using H2O as the solvent (Table 1) [22]. It should be noted that, no evidence for the formation of peresters 5 and 6 was observed under the present reaction conditions (entries 1–4). The use of 50 mol% of nBu4NBr significantly decreased the yield of product (entry 5). Other bromides such as Et4NBr, NBS and NaBr were found to be less effective than nBu4NBr (entries 6–8). We also examined other catalysts such as Lewis and protic acids. However, they proved to be less efficient or ineffective (entries 9–14).
|
|
Table 1 Screening of the reaction conditions.a |
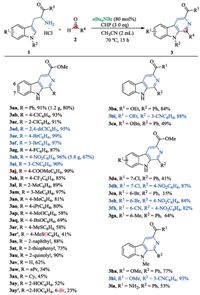
With optimized conditions in hand (Table 1, entry 4), we next studied the scope and limitations of the β-carboline synthesis by first varying the aldehyde component in the reaction with tryptophan methyl ester hydrochloride 1a (Scheme 1, 3aa-ay). Benzaldehydes with electron-withdrawing (e.g., -Hal, -NO2, -CN, -CO2Me, -CF3) and moderately electron-donating groups (-CH3, -iPr) were well-tolerated, leading to the desired products 3ab-ao in high yields (80%–99%). The moderate yields were observed when 4-methoxy and 4-benzyloxy benzaldehydes were subjected to the reaction (3ap and 3aq). 4-Methylthiobenzaldehyde was converted to the corresponding β-carboline 3ar in 58% yield. Interestingly, this annulation was accompanied by a concomitant oxidation of thioether, resulting in the formation of sulfoxide substituted β-carboline 3ar' in 41% yield. The reactions of 2- naphthaldehyde, 2-thiophenecarbaldehyde and 2-pyridinecarbaldehyde occurred smoothly to afford the corresponding products 3as-au in good yields. In the case of formaldehyde, the reaction gave the desired product 3av in 62% yield. Its structure was confirmed unambiguously by single crystal X-ray diffraction (Supporting information). Unfortunately, the aliphatic aldehydes showed low reactivity, giving products 3aw and 3ax in low yields and large amounts of recovered starting material. Surprisingly, when the annulation was attempted with salicylaldehyde, an unexpected bromination reaction of product 3ay occurred to give 6-bromo-β-carboline 3ay' in 25% yield.
|
Download:
|
| Scheme 1. Scope of the substrates. Reaction conditions: 1 (0.25 mmol), 2 (1.5 equiv., 0.38 mmol), nBu4NBr (80 mol%), CHP (3.0 equiv.), under air; isolated yields. | |
We continued the studies by varying the tryptophan ester component. The ethyl and benzyl esters of tryptophan were both compatible with the reaction conditions (3ba, 3bi and 3ca). When benzaldehyde 2a was used as the aldehyde component, the groups in the 6-, or 7-position of indole ring had a marked effect on the efficiency of the reaction. They decreased the yields of products regardless whether they were EWG or EDG (3da, 3ea and 3ga). In contrast, the high reactive 4-nitrobenzaldehyde reacted with these tryptophan methyl esters to afford β-carbolines in good yields (3dh, 3eh and 3fh). The present annulation was also attempted on N-methyl tryptophan methyl ester 1 h. The desired products 3ha and 3hi were obtained in good yields. We finally found that even a primary amide group could be tolerated under the reaction conditions (3ia). In the cases of 3ad-af, 3ah, 3ai, 3bi, 3dh-fh and 3hi, they precipitated from the reaction mixture as crystalline complexes at the end of the reaction (Scheme 1, blue colour). The analytically pure products could be readily isolated by filtration. Moreover, the present synthesis is very practical and allows the preparation of β-carbolines on gram-scale (3aa, 1.2 g, 80% yield; 3 ah, 5.8 g, 67% yield). It should be noted that the present reaction could not be applicable to the 5 or 8-substituted tryptophan esters due to the production of complex product mixtures.
The control experiments were performed to explore the mechanism of the cycloaromatization after we established the substrate scope (Scheme 2). The addition of radical inhibitors TEMPO or BHT had no effect on the yield of the 3aa (reaction 1). When bromine-free phase transfer catalyst such as (nBu4N)2SO4 was used in place of nBu4NBr, 3aa was not observed (reaction 2). To explore the potential bromine species, Br2 was further used as the mediator. The reactions afforded complex mixtures containing trace amounts of 3aa (reaction 3).

|
Download:
|
| Scheme 2. Control experiments. | |
Based on our results and previous reports [23], a mechanistic pathway is proposed in Scheme 3. First, the reaction of 1a with 2a yields an imine A, which undergoes bromination with Br+ to afford N-bromoiminium B [23b,24]. The electrophilicity of imine B is enhanced by the halogen bond interaction [24a]. Second, the intramolecular cyclization of B yields C, which converts into D through the elimination of proton. Third, the elimination of HBr from D yields the partially aromatized intermediate E. Finally, the bromination of E takes place to give intermediate F, followed by elimination of HBr from G to afford product 3aa [9g,9h].

|
Download:
|
| Scheme 3. Proposed mechanism. | |
In summary, we have developed a practical and scalable method for the synthesis of β-carbolines. The protocol uses readily available tryptophans and aldehydes as starting materials, and nBu4NBr as the environmentally friendly mediator, providing target products in high yields. Considering the valuable structure of the products, simple isolation procedure and acid/metal-free conditions, this methodology should find broad applications.
AcknowledgmentsThe authors thank the National Natural Science Foundation of China (Nos. 21172120 and 21472093) and Tianjin Municipal Science and Technology Commission (No. 14JCYBJC20600) for financial support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.07.001.
| [1] |
(a) R.H. Cao, W.L. Peng, Z.H. Wang, et al., Curr. Med. Chem. 14 (2007) 479-500; (b) S. Lancianesi, A. Palmieri, M. Petrini, Chem. Rev. 114 (2014) 7108-7149; (c) A.S. Nagle, S. Khare, A.B. Kumar, et al., Chem. Rev. 114 (2014) 11305-11347; (d) D. Gema, P.C. Javier, Eur. J. Org. Chem. 2011 (2011) 7243-7253; (e) G.J. Zhang, F. Hu, H. Jiang, et al., Phytochemistry 145 (2018) 68-76; (f) R.P.O. Venkataramana, M. Hridhay, K. Nikhil, et al., Bioorg. Med. Chem. Lett. 28 (2018) 1278-1282; (g) N. Devi, S. Kumar, S.K. Pandey, et al., Asian J. Org. Chem. 7 (2018) 6-36; (h) J.G. Luo, L.H. Cao, L.Y. Kong, Chin. Chem. Lett. 23 (2012) 1385-1388; (i) Q.Z. Wang, J.Y. Liang, X. Feng, Chin. Chem. Lett. 21 (2010) 596-599. |
| [2] |
(a) A.G. Shilabin, N. Kasanah, B.L. Tekwani, et al., J. Nat. Prod. 71 (2008) 1218-1221; (b) J.D. Winkler, A.T. Londregan, M.T. Hamann, Org. Lett. 8 (2006) 2591-2594. |
| [3] |
(a) Y. Boursereau, I. Coldham, Bioorg. Med. Chem. Lett. 14 (2004) 5841-5844; (b) H. Guan, H. Chen, W. Peng, et al., Eur. J. Med. Chem. 41 (2006) 1167-1179; (c) M.A. Rashid, K.R. Gustafson, M.R. Boyd, J. Nat. Prod. 64 (2001) 1454-1456; (d) M.R. Prinsep, J.W. Blunt, M.H.G. Munro, J. Nat. Prod. 54 (1991) 1068-1076; (e) J.M. Yang, Y.H. Zhu, S. Chen, et al., MedChemComm 9 (2018) 100-107; (f) S.U. Dighe, S. Khan, I. Soni, et al., J. Med. Chem. 58 (2015) 3485-3499; (g) A. Kamal, V. Srinivasulu, V.L. Nayak, et al., ChemMedChem 9 (2014) 2084-2098; (h) Z.G. Li, G.Q. Dong, S.Z. Wang, et al., Chin. Chem. Lett. 26 (2015) 267-271; (i) L. Liu, Y.Y. Xu, Z.Q. Yang, et al., Chin. Chem. Lett. 23 (2012) 1230-1232; (j) K. Fang, G.Q. Dong, H. Gong, et al., Chin. Chem. Lett. 25 (2014) 978-982. |
| [4] |
(a) J.G. Tang, Y.H. Wang, R.R. Wang, et al., Chem. Biodivers. 5 (2008) 447-460; (b) Y.H. Wang, J.G. Tang, R.R. Wang, et al., Biochem. Biophys. Res. Commun. 355 (2007) 1091-1095; (c) X. Yu, W. Lin, J. Li, et al., Bioorg. Med. Chem. Lett. 14 (2004) 3127-3130. |
| [5] |
(a) T.J. Hagen, P. Skolnick, J.M. Cook, J. Med. Chem. 30 (1987) 750-753; (b) W.E. Müller, K.J. Fehske, H.O. Borbe, et al., Pharmacol. Biochem. Behav. 14 (1981) 693-699. |
| [6] |
(a) Y. Im, J.Y. Lee, Chem. Commun. 49 (2013) 5948-5950; (b) B.K. Paul, N. Ghosh, S. Mukherjee, RSC Adv. 6 (2016) 9984-9993; (c) S. Swami, D. Behera, A. Agarwala, et al., New J. Chem. (2018) 10317-10326. |
| [7] |
(a) W.M. Whaley, T.R. Govindachari, The Pictet-Spengler Synthesis of Tetrahydroisoquinolines and Related Compounds, Organic Reactions, John Wiley & Sons, Inc., 2004; (b) E.D. Cox, J.M. Cook, Chem. Rev. 95 (1995) 1797-1842; (c) R.N. Rao, B. Maiti, K. Chanda, ACS Comb. Sci. 19 (2017) 199-228; (d) V. Gobe, V. Gandon, X. Guinchard, Adv. Synth. Catal. 360 (2018) 1280-1288; (e) C. Glenn, C.B. Jan, Eur. J. Org. Chem. 2004 (2004) 1286-1297; (f) Y.N. Sun, C.L. Wang, N. Zhang, et al., Chin. Chem. Lett. 25 (2014) 1503-1506; (g) P.Y. Zhang, S.B. Wan, S.M. Ren, et al., Chin. Chem. Lett. 21 (2010) 1307-1309; (h) P.Y. Zhang, J.L. Wang, S.B. Wan, et al., Chin. Chem. Lett. 21 (2010) 889-891. |
| [8] |
W.M. Whaley, T.R. Govindachari, The Preparation of 3, 4-Dihydroisoquinolines and Related Compounds by the Bischler-Napieralski Reaction, Organic Reactions, John Wiley & Sons, Inc., 2004.
|
| [9] |
(a) D. Singh, P. Sharma, R. Kumar, et al., Asian J. Org. Chem. 7 (2018) 383-394; (b) D. Singh, C.K. Hazra, C.C. Malakar, et al., ChemistrySelect 3 (2018) 4859-4864; (c) J. Kovvuri, B. Nagaraju, V.L. Nayak, et al., Eur. J. Med. Chem.143 (2018) 1563-1577; (d) D. Singh, V. Kumar, N. Devi, et al., Adv. Synth. Catal. 359 (2017) 1213-1226; (e) K.L. Manasa, Y. Tangella, G. Ramu, et al., ChemistrySelect 2 (2017) 9162-9167; (f) C.E.P. Galvis, V.V. Kouznetsov, Synthesis 49 (2017) 4535-4561; (g) S. Hati, S. Sen, Tetrahedron Lett. 57 (2016) 1040-1043; (h) R. Meesala, A.S.M. Arshad, M.N. Mordi, et al., Tetrahedron 72 (2016) 8537-8541; (i) A. Kamal, Y. Tangella, K.L. Manasa, et al., Org. Biomol. Chem.13 (2015) 8652-8662; (j) A. Kamal, M. Sathish, A.V.G. Prasanthi, et al., RSC Adv. 5 (2015) 90121-90126; (k) A. Kamal, M.P. Narasimha Rao, P. Swapna, et al., Org. Biomol. Chem. 12 (2014) 2370-2387. |
| [10] |
(a) S. Ding, Z. Shi, N. Jiao, Org. Lett. 12 (2010) 1540-1543; (b) S. Tang, J. Wang, Z. Xiong, et al., Org. Lett. 19 (2017) 5577-5580; (c) S.P. Mulcahy, J.G. Varelas, Tetrahedron Lett. 54 (2013) 6599-6601; (d) S. Dhara, R. Singha, A. Ahmed, et al., RSC Adv. 4 (2014) 45163-45167; (e) Q. Yan, E. Gin, M.G. Banwell, et al., J. Org. Chem. 82 (2017) 4328-4335; (f) S. Dhiman, U.K. Mishra, S.S.V. Ramasastry, Angew. Chem. Int. Ed. 55 (2016) 7737-7741; (g) X. Pan, T.D. Bannister, Org. Lett. 16 (2014) 6124-6127; (h) T.T. Wang, D. Zhang, W.W. Liao, Chem. Commun. 54 (2018) 2048-2051. |
| [11] |
B.A. Dalvi, P.D. Lokhande, Tetrahedron Lett. 59 (2018) 2145-2149. DOI:10.1016/j.tetlet.2018.01.061 |
| [12] |
F. Nissen, V. Richard, C. Alayrac, et al., Chem. Commun. 47 (2011) 6656-6658. DOI:10.1039/c1cc11298h |
| [13] |
(a) J.G. Varelas, S. Khanal, M.A. O'Donnell, et al., Org. Lett.17 (2015) 5512-5514; (b) N.J. Webb, S.P. Marsden, S.A. Raw, Org. Lett. 16 (2014) 4718-4721. |
| [14] |
(a) G. Verniest, D.England, N.DeKimpe, et al., Tetrahedron 66 (2010)1496-1502; (b) B.V. Subba Reddy, M. Rajashekhar Reddy, S. Yarlagadda, et al., J. Org. Chem. 80 (2015) 8807-8814. |
| [15] |
P.C. Too, S.H. Chua, S.H. Wong, et al., J. Org. Chem. 76 (2011) 6159-6168. DOI:10.1021/jo200897q |
| [16] |
Y.P. Zhu, M.C. Liu, Q. Cai, et al., Chem. Eur. J. 19 (2013) 10132-10137. DOI:10.1002/chem.201301734 |
| [17] |
(a) M. Uyanik, K. Ishihara, ChemCatChem 4 (2012) 177-185; (b) P. Finkbeiner, B.J. Nachtsheim, Synthesis 45 (2013) 979-999; (c) X.F. Wu, J.L. Gong, X.X. Qi, Org. Biomol. Chem. 12 (2014) 5807-5817; (d) D. Liu, A.W. Lei, Chem. Asian J. 10 (2015) 806-823. |
| [18] |
Z. Shi, F. Glorius, Chem. Sci. 4 (2013) 829-833. DOI:10.1039/C2SC21823B |
| [19] |
H. Rao, X. Ma, Q. Liu, et al., Adv. Synth. Catal. 355 (2013) 2191-2196. DOI:10.1002/adsc.201300488 |
| [20] |
B. Wang, H.N.C. Wong, Bull. Chem. Soc. Jpn. 91 (2018) 710-719. DOI:10.1246/bcsj.20170393 |
| [21] |
(a) Z.Y. Yang, T. Tian, Y.F. Du, et al., Chem. Commun. 53 (2017) 8050-8053; (b) H. Wang, Z. Wang, Y.L. Wang, et al., Org. Lett. 19 (2017) 6140-6143; (c) T. Lu, Y.T. Jiang, F.P. Ma, et al., Org. Lett. 19 (2017) 6344-6347. |
| [22] |
W. Wei, C. Zhang, Y. Xu, et al., Chem. Commun. 47 (2011) 10827-10829. DOI:10.1039/c1cc14602e |
| [23] |
(a) S. Guha, I. Kazi, A. Nandy, et al., Eur. J. Org. Chem. (2017) 5497-5518; (b) X. Wang, D. Xu, C. Miao, et al., Org. Biomol. Chem. 12 (2014) 3108-3113. |
| [24] |
(a) Y. Wei, S. Lin, F. Liang, et al., Org. Lett. 15 (2013) 852-855; (b) Y. Wei, F. Liang, X. Zhang, Org. Lett. 15 (2013) 5186-5189. |
 2019, Vol. 30
2019, Vol. 30 


