b State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing 100191, China;
c State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China
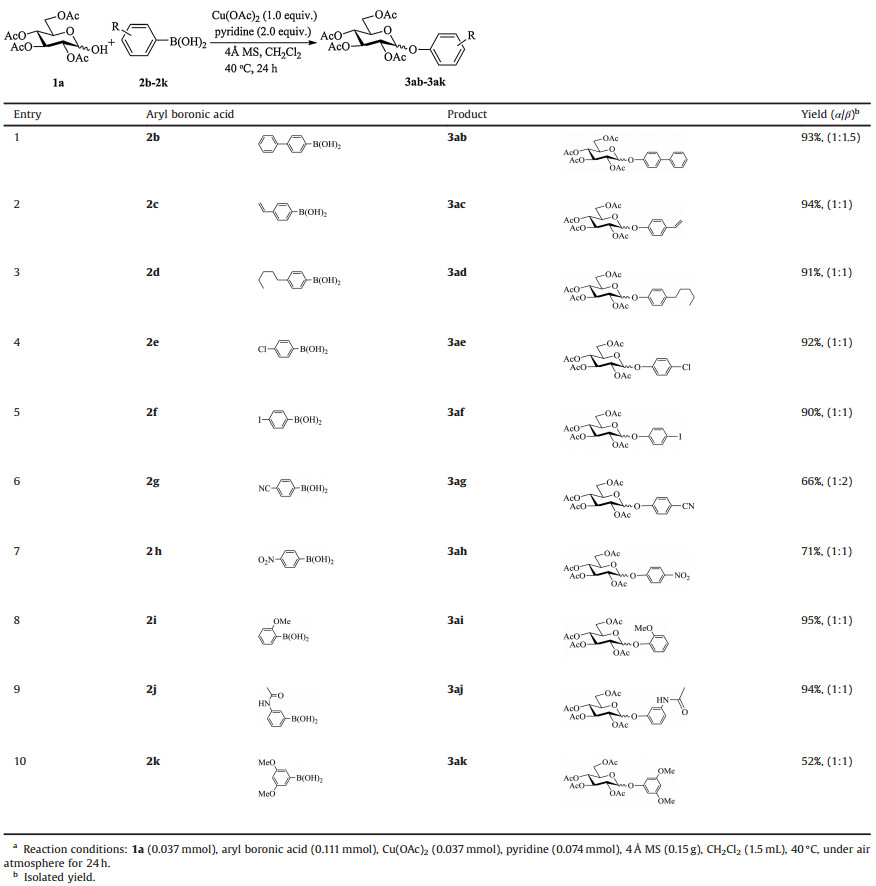
Phenolic glycosides such as vancomycin [1], seenoside A [2] and camellianin B [3] (Scheme 1a), refer to molecules containing a sugar unit bound to a phenol aglycone. These compounds are widely distributed in nature and play numerous important roles in living organisms. Phenolic glycosides have also received special attention owing to their vital pharmaceutical potentials, such as antitumor [4], antidiabetic [5] and anti-inflammatory effects [6]. A major obstacle in the development of pharmacological characterization of phenolic glycosides is their extremely trace content in natural sources.
|
Download:
|
| Scheme 1. Phenolic glycosides and their synthetic methods. | |
Chemical synthesis enables facile access to phenolic glycosides in pure and large quantities. There has been a growing emphasis on investigating the synthesis of phenolic glycosides. A great deal of efforts has been devoted to the development of effective methods for the synthesis of phenolic glycosides. O-Glycosylation of phenols is the main strategy for the formation of these compounds. A large variety of glycosyl donors such as glycosyl halides [7], glycosyl trichloroacetimidates [8], glycosyl N-phenyl trichloroacetimidates [9], glycosyl acetates [10], thioglycosides [11], alkynyl glycosides [12], 1, 2-anhydrosugar [13], and hemiacetals [14], have been used for the assembly of phenolic glycosides either through an SN2 type mechanism under basic conditions or through an SN1 type mechanism under acidic conditions (Scheme 1b). However, when using these methods, the anomerization, formation of C-glycoside by-products, and low functional-group tolerance usually hamper the versatility and utility of phenolic glycoside synthesis.
O-Arylation of hemiacetals represents another route to afford phenolic glycosides. The Olofsson group developed a novel method for anomeric O-arylation using bench-stable iodonium (Ⅲ) reagents [15]. Xiao group revealed a new coupling reaction of sugar lactols with aryl bromides to form phenolic glycosides via dual photoredox/Ni catalysis [16]. As part of our continuous studies on the preparation of carbohydrate derivatives by the coupling reactions with aryl boronic acids [17], we herein report the synthesis of phenolic glycosides via copper-mediated O-arylation of lactols with aryl boronic acids (Scheme 1c).
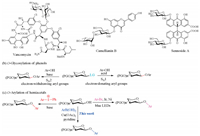
We started our investigations on the coupling reaction of 2, 3, 4, 6-tetra-O-acetyl-D-glucopyranose (1a) with commercially available 4-methoxyphenylboronic acid (2a) (Table 1 and Tables S1-S3 in Supporting information). We anticipated that a stoichiometric amount of copper salt would be necessary for the formation of the C–O bond. Indeed, the desired product 3aa was obtained in 8% isolated yield by the use of CuCl2 (1.0 equiv.) in the presence of pyridine (3.0 equiv.) (entry 1). The reaction appeared completely inert to Cu(OTf)2, CuSO4·5H2O, CuCl and CuBr (entries 2–5). Gratifyingly, the use of Cu(OAc)2 instead of CuCl2 afforded the desired product 3aa in 62% yield (entry 6). Increasing the reaction temperature to 40 ℃ improved the reaction efficiency (Table S3). It was observed that pyridine was the proper base and the amount of pyridine also influenced the reaction yield (the best amount was 2.0 equiv.) (entries 7–11 and 14–15, and Tables S1 and S3). Further studies indicated that dichloromethane would be the best solvent (Table S2). The omission of molecular sieves failed to give the product (entry 16, Table S4 in Supporting information). Therefore, the optimized conditions are as follows: lactol (1.0 equiv.), aryl boronic acid (3.0 equiv.), Cu(OAc)2 (1.0 equiv.), pyridine (2.0 equiv.) and activated 4 Å molecular sieves, in CH2Cl2 at 40 ℃ for 24 h (entry 15).
|
|
Table 1 Screening of optimal conditions.a |
Under the optimized conditions, the reaction of lactol 1a with different aryl boronic acids were surveyed, as shown in Table 2. The results showed that aryl boronic acids bearing both electrondonating substituents and electron-withdrawing substituents underwent the reaction smoothly, affording the phenolic glycosides in moderate to excellent yields. The reaction of ortho-, meta-and para-substituted aryl boronic acid substrates gave good yields (entries 8 and 9). Interestingly, the reaction of vinyl-, chloro- and iodo-substituted aryl boronic acids with compound 1a also proceeded very well, providing the desired coupled products, which could be used for further transformations. The reaction of disubstituted 3, 5-dimethoxyphenylboronic acid gave the desired product 3ak in moderate yield (52%, entry 10).
|
|
Table 2 The scope of aryl boronic acids.a |
Next, the scope of lactols was explored under the optimized conditions and the results are summarized in Table 3. The reaction of 2, 3, 4, 6-tetra-O-acetyl-D-galactose (1b), 2, 3, 4, 6-tetraO-acetyl-D-mannose (1c), 2, 3, 4-tri-O-acetyl-L-arabinose (1d) and 2, 3, 4-tri-O-acetyl-D-xylose (1e) with both electron-rich p-methox-ybenzeneboronic acid (2a) and electron-deficient p-nitrobenze-neboronic acid (2 h), proceeded in good yields (entries 1–5). 2, 3, 4-Tri-O-benzyl-L-fucose (1f) also tolerated in this transformation, generating product 3fa in 65% isolated yield (entry 6). Similarly, the benzylated/benzoylated counterpart of glucose/galactose/mannose, underwent the reaction smoothly in satisfactory yields (entries 7–16). It is noteworthy that the reaction of the mannosederived 1c and 1k provided an exclusive α-product (entries 3, 15 and 16). In all the reactions, no C-glycoside byproducts were detected. Most of the produced phenolic glycosides were lack of stereoselectivity, and the anomeric ratio of the obtained glycosides was not identical with that of the corresponding lactols in CDCl3. It might be because that the anomeric ratio of lactols could vary as the change of temperature, basicity, and solvent.
|
|
Table 3 The scope of lactols.a |
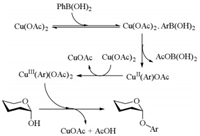
Based on the literature report [18], a plausible reaction mechanism is depicted in Scheme 2. Initially, the aryl boronic acid reacts with Cu (OAc)2 to generate CuⅡ(Ar)OAc, which is oxidized with another Cu (OAc)2 to form CuⅢ(Ar)(OAc)2. Finally, the ligand exchange and the latter reductive elimination furnishes the phenolic glycosides.
|
Download:
|
| Scheme 2. The proposed mechanism for the O-arylation of lactols. | |
In conclusion, we have developed an efficient and practical copper-mediated coupling reaction of lactols with aryl boronic acids for the preparation of phenolic glycosides. The O-arylation of the anomeric oxygen in sugar moiety proceeded in moderate to excellent yields under mild reaction conditions. A broad range of lactols and aryl boronic acids with various functional groups were tolerated. The disclosed approach may find wide applications in the synthesis of many phenolic glycosides with biological importance.
AcknowledgmentsThis work was financially supported by the National Key R & D Program of China (No. 2018YFA0507602), the National Natural Science Foundation of China (Nos. 21572012, 21772006) and the State Key Laboratory of Drug Research (No. SIMM1803KF-02).
Appendix A. Supplementary dataSupplementary material related to this article can befound, inthe online version, at doi:https://doi.org/10.1016/j.cclet.2019.06.014.
| [1] |
D. Kahne, C. Leimkuhler, L. Wei, et al., Chem. Rev. 105 (2005) 425-448. DOI:10.1021/cr030103a |
| [2] |
S.Y. Lee, W. Kim, Y.G. Lee, et al., Pharmacol. Res. 119 (2017) 422-430. DOI:10.1016/j.phrs.2017.03.003 |
| [3] |
W. Li, R.J. Dai, Y.H. Yu, et al., Biol. Pharm. Bull. 30 (2007) 1123-1129. DOI:10.1248/bpb.30.1123 |
| [4] |
(a) R. Riccio, K. Nakanishi, J. Org. Chem. 47 (1982) 4589-4592; (b) B. La Ferla, C. Airoldi, C. Zona, et al., Nat. Prod. Rep. 28 (2011) 630-648; (c) C. Mendez, J. Gonzalez-Sabin, F. Moris, et al., Planta Med. 81 (2015) 1326-1338; (d) H. Murase, T. Noguchi, S. Sasaki, Bioorg. Med. Chem. Lett. 28 (2018) 1832-1835; (e) B.T. Scroggins, J. Burkeen, A.O. White, et al., Int. J. Radiat. Oncol. Biol. Phys. 100 (2018) 344-352; (f) N. Tatematsu, Y. Waguri-Nagaya, Y. Kawaguchi, et al., Mod. Rheumatol. 28 (2018) 495-505. |
| [5] |
(a) S. Burda, W. Oleszek, J. Agric. Food Chem. 49 (2001) 2774-2779; (b) Y.S.R. Elnaggar, E.M.M. Shehata, S. Galal, Nanomedicine 12 (2017) 893-910. |
| [6] |
C.S. Kim, L. Subedi, K.J. Park, et al., Fitoterapia 106 (2015) 147-152. DOI:10.1016/j.fitote.2015.08.013 |
| [7] |
(a) W. Koenigs, E. Knorr, Ber. Dtsch. Chem. Ges. 34 (1901) 957-981; (b) K. Mohri, Y. Watanabe, Y. Yoshida, et al., Chem. Pharm. Bull. 51 (2003) 1268-1272. |
| [8] |
(a) R.R. Schmidt, J. Miche, Angew. Chem. Int. Ed. 19 (1980) 731-732; (b) D.A. Burnett, M.A. Caplen, M.S. Domalski, et al., Bioorg. Med. Chem. Lett. 12 (2002) 311-314; (c) P.W. Qin, J. Wang, H. Wang, et al., J. Agric. Food Chem. 62 (2014) 4521-4527. |
| [9] |
(a) M. Li, X.W. Han, B. Yu, J. Org. Chem. 68 (2003) 6842-6845; (b) C. Menozzi-Smarrito, C.C. Wong, W. Meinl, et al., J. Agric. Food Chem. 59 (2011) 5671-5676. |
| [10] |
Y.S. Lee, E.S. Rho, Y.K. Min, et al., J. Carbohydr. Chem. 20 (2001) 503-506. DOI:10.1081/CAR-100106933 |
| [11] |
(a) S.G. Duron, T. Polat, C.H. Wong, Org. Lett. 6 (2004) 839-841; (b) G.J. Liu, C.Y. Li, X.T. Zhang, et al., Chin. Chem. Lett. 29 (2018) 1-10. |
| [12] |
(a) Y. Hu, Y.H. Tu, D.Y. Liu, et al., Org. Biomol. Chem. 14 (2016) 4842-4847; (b) J.F. Zhou, X. Chen, Q.B. Wang, et al., Chin. Chem. Lett. 21 (2010) 922-926. |
| [13] |
C.F. Liu, D.C. Xiong, X.S. Ye, Tetrahedron Lett. 57 (2016) 1372-1374. DOI:10.1016/j.tetlet.2016.02.059 |
| [14] |
(a) W.R. Roush, R.A. Hartz, D.J. Gustin, J. Am. Chem. Soc.121 (1999) 1990-1991; (b) L. Petersen, K.J. Jensen, J. Org. Chem. 66 (2001) 6268-6275; (c) J.P. Issa, C.S. Bennett, J. Am. Chem. Soc. 136 (2014) 5740-5744. |
| [15] |
(a) G.L. Tolnai, U.J. Nilsson, B. Olofsson, Angew. Chem. Int. Ed. 55 (2016) 11226[-11230; (b) N. Lucchetti, R. Gilmour, Chem. -Eur. J. 24 (2018) 16266-16270. |
| [16] |
H. Ye, C. Xiao, Q.Q. Zhou, et al., J. Org. Chem. 83 (2018) 13325-13334. DOI:10.1021/acs.joc.8b02129 |
| [17] |
(a) D.C. Xiong, L.H. Zhang, X.S. Ye, Org. Lett. 11 (2009) 1709-1712; (b) C.F. Liu, D.C. Xiong, X.S. Ye, J. Org. Chem. 79 (2014) 4676-4686; (c) S.B. Tang, Q.N. Zheng, D.C. Xiong, et al., Org. Lett. 20 (2018) 3079-3082. |
| [18] |
(a) S.V. Ley, A.W. Thomas, Angew. Chem. Int. Ed. 42 (2003) 5400-5449; (b) A.E. King, T.C. Brunold, S.S. Stahl, J. Am. Chem. Soc. 131 (2009) 5044-5045. |
 2019, Vol. 30
2019, Vol. 30