b Shanghai-Hong Kong Joint Laboratory in Chemical Synthesis, Shanghai Institute of Organic Chemistry, The Chinese Academy of Sciences, Shanghai 200032, China
Henry reaction (nitroaldol condensation) is a versatile carbon–carbon bond forming reaction to synthesize important building blocks including β-hydroxynitroalkanes, amino-alcohols and α-hydroxy carboxylic acids [1]. Since the seminal report of rare earth metal-BINOL complexes in 1992 for catalytic asymmetric Henry reaction by Shibasaki [2], significant efforts have been devoted to the development of transition-metal catalyzed enantioselective Henry reaction with various catalytic complexes [1c, 1d, 3]. In the field of organocatalysis, the first example was published in 1994 by Nájera employing chiral guanidines [4]. Subsequently, guanidine derived catalysts with various skeletons were designed to improve the enantioselectivities of the reaction involving nitroalkanes and aldehydes [5]. On the other hand, Corey pioneered the use of chiral quaternary ammonium salts in stereoselective Henry reaction in the synthesis of amprenavir [6]. In 2003, the prominent Maruoka catalyst was reported to control efficiently the stereochemical outcomes of this transformation [7]. Apart from phase transfer catalysts, cinchona alkaloids and thioureas were demonstrated to be effective catalysts in terms of both efficiency and enantioselectivity [8].
Besides aldehydes as substrates for Henry reaction [9], ketones were less explored due to its diminished reactivity. However, the resulted chiral tertiary alcohols bearing quaternary stereocenters were very attractive for the synthesis of bio-active molecules. In 2006, Deng reported a cinchona alkaloids-catalyzed Henry reaction with α-ketoesters bearing alkenyl, aryl and alkyl groups [10]. Nagasawa explored the same reaction using guanidine-derived thiourea catalysts [5b-d]. Later, the vicinal activated ketones of α-ketophosphonates [11], trifluoromethylketones [12], isatins [13] and 1H-pyrrole-2, 3-diones [14] were exploited in the organocatalytic enantioselective Henry reaction with nitromethane by several research groups (Scheme 1, a).

|
Download:
|
| Scheme 1. Asymmetric Henry reaction and chiral iminophophorane catalysis. | |
On the other hand, recent years has witnessed a variety of alkyne transformations, such as the Pauson-Khand reaction [15], enyne cycloisomerization [16], and heterocyclization [17]. The large number of such transformations indicate that the alkyne moiety is a useful carbon-based functional group. In this regard, α-keto esters with an alkynyl moiety were reported to be an excellent substrate with asymmetric direct aldol reaction [18]. However, to the best of our knowledge, asymmetric Henry reaction of nitromethane with α, β-alkynyl ketoesters has not yet been reported. In a continuation of our recent effort as well as to expand the reaction scope on a class of air and moisture stable chiral iminophosphoranes as organocatalysts (Scheme 1, b) [19], we herein report the use of iminophosphorane catalysts for asymmetric Henry reaction of nitromethane and α, β-alkynyl ketoesters, resulting in the formation of chiral tertiary alcohols bearing a alkyne moiety (Scheme 1, c).
In connection with our previous studies concerning asymmetric Henry reaction and chiral iminophosphorane catalysis [20-21], we initially conducted the Henry reaction of α-keto ester 1a with nitromethane 2a by using toluene as solvent at room temperature to determine the catalytic activity and enantioselectivity of the iminophosphorane catalysts.
As shown in Table 1, it was found that the reaction proceeded smoothly for 9 h in the presence of iminophosphoranes 4 as organocatalysts to furnish the desired products in moderate yields and low enantioselectivities of 2%–26%. Catalyst 4b with bulky tert-butyl substituents displayed the best results in terms of both activity and selectivity. Prolonged reaction time for 22 h could significantly enhance the reaction yield in 84% with 25% ee (entry 9). With catalyst 4b in hand, we then screened solvents to establish the optimum reaction conditions. The reaction underwent smoothly in aprotic solvents such as dichloromethane (DCM), tetrahydrofuran (THF), diethyl ether (Et2O) and n-pentane. However, enantioselectivities still remained very low (0–27% ee, entries 10–13). When screening the polar solvent of acetonitrile (CH3CN), dimethylsulfoxide (DMSO), dioxane and ethyl acetate (EtOAc), we were pleased to find out that the enantioselectivity was enhanced to 54% in EtOAc with an excellent yield of 90% (entry 16). Moreover, better enantiomeric excess value of 68% was achieved in dioxane as solvent although the yield was only 43%. Hence, a mixture of co-solvent dioxane/EtOAc was assessed. Gratifyingly, dioxane/EtOAc (1/1) was found to be the most suitable co-solvent for the reaction, affording the product in 71% yield and 66% ee at room temperature. Lowering the reaction temperature to -30 ℃ led an improvement on enantioselectivity (82% ee, entry 19). An attempt to increase the catalyst loading turned out to improve both productivity and enantio-discrimination with 20 mol% catalyst loading (93% yield with 87% ee). However, further reducing the reaction temperature to -40 ℃ resulted in a dramatically sluggish reaction rate.
|
|
Table 1 Screening the reaction conditions for asymmetric Henry reaction.a |
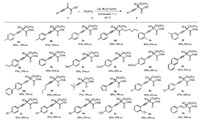
Next, we started to investigate the substrate scope of α-keto ester 1 with nitromethane in the asymmetric Henry reaction in the presence of 20 mol% iminophosphorane catalyst 4b at -30 ℃ by using dioxane/EtOAc (1/1) as solvent. As summarized in Scheme 2, firstly, the enantiomer of catalyst 4b' was also utilized in the reaction, 3a' was obtained in 86% yield with 78% ee. As can be seen, all substrates 1 bearing various functional groups (R1 and R2) on the α, β-alkynyl ketoesters skeleton could be converted smoothly into the corresponding products 3. When R2 were alkyl groups of methyl, ethyl, isopropyl, or ether chain with increasing steric hindrance, the ee values of the corresponding products slightly decreased (83%–93% yield, 44%–87% ee; 3a-d). Employing substituted phenyl groups (R1) of the alkyne moiety on the substrates, the reaction afforded a range of products 3e-m with 86%–99% yield and 55%–84% ee. Moreover, when R1 are alkyl groups, the reaction gave 3n and 3o in 84% yield with 64% ee and 77% yield with 76% ee, respectively. Varying R1 with halogen, cyano, trifluoromethyl or nitro groups on the aryl ring also afforded moderate enantioselectivities even with excellent reaction efficiency (82%–99% yield, 20%–62% ee; 3q-3w). Notably, when R1 was thiophene, Henry reaction product 3p was furnished in 88% yield with 78% ee. Furthermore, α-keto ester with an alkenyl moiety was also tried in this reaction, the product 3x was obtained in 42% yield with only 34% ee.
|
Download:
|
| Scheme 2. Scope of asymmetric Henry reaction. All reactions were carried out with 1 (0.1 mmol, 1.0 equiv.), 2 (1.0 mmol, 10.0 equiv.) and catalyst 4b (0.02 mmol, 20 mol%) in EA and dioxane (1 mL/1 mL) at -30 ℃ under argon. | |
Noteworthy is that the absolute configuration of optically active 3s bearing a bromine atom on the phenyl ring was established to be S by a single-crystal X-ray crystallographic analysis with CCDC 1907837 (Fig. 1, left). However, it is interesting to note that products 3b-d and 3t with steric R2 showed an opposite optical rotations to the product 3a. Fortunately, a single crystal of 3t was also obtained for X-ray crystallographic analysis (CCDC 1907838), whose absolute configuration was unambiguously determined to be S (Fig. 1, right). Thus, the Henry reaction of products 3 from this reaction were assumed to have the same (S)-configuration as those of 3s and 3t.

|
Download:
|
| Fig. 1. X-ray crystal structures of 3s (left) and 3t (right); the thermal ellipsoids at the probability level is 30% for the crystal structures. | |
In conclusion, by using iminophosphoranes as organocatalysts with dioxane/EtOAc as solvent, the desired Henry reaction products of optically active β-nitro-substituted tertiary alcohols were obtained in moderate to excellent yields with medium levels of enantiomeric excesses (up to 87% ee). This is the first report on the use α, β-alkynyl ketoesters in asymmetric Henry reaction with nitromethane. Studies on the catalytic potentials of these chiral phosphorus-based catalysts are in progress in our laboratories.
AcknowledgmentsThis work is dedicated to Professor Henry N. C. Wong for his significant contribution to the development of organic chemistry in China. This work was supported by grants from National Key Program (No. 2016YFA0200302, Study on application and preparation of aroma nanocomposites), National Natural Science Foundation of China (NSFC, No. 21472213) and Croucher Foundation (Hong Kong) in the form of a CAS-Croucher Foundation Joint Laboratory Grant. We also thank Professor Henry N. C. Wong for helpful discussion and generous support.
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.042.
| [1] |
(a) L. Henry, Compt. Rend. Hebd. Seances Acad. Sci. 120 (1895) 1265-1267; (b) L. Henry, Bull. Soc. Chim. Fr. 13 (1895) 999-1003; (c) S. Zhang, Y. Li, Y. Xu, Z. Wang, Chin. Chem. Lett. 29 (2018) 873-883; (d) J. Boruwa, N. Gogoi, P.P. Saikia, N.C. Barua, Tetrahedron: Asymmetry 17 (2006) 3315-3326. |
| [2] |
(a) H. Sasai, T. Suzuki, S. Arai, T. Arai, M. Shibasaki, J. Am. Chem. Soc. 114 (1992) 4418-4420; (b) H. Sasai, T. Suzuki, N. Itoh, et al., J. Am. Chem. Soc. 115 (1993) 10372-10373. |
| [3] |
(a) F.A. Luzzio, Tetrahedron 57 (2001) 915-945; (b) C. Palomo, M. Oiarbide, A. Laso, Eur. J. Org. Chem. (2007) 2561-2564. |
| [4] |
R. Chinchilla, C. Nájera, Sánchez-Agulló P., Tetrahedron:Asymmetry 5 (1994) 1393-1402. |
| [5] |
(a) D. Ma, Q. Pan, F. Han, Tetrahedron Lett. 43 (2002) 9401-9403; (b) X.S. Li, Z.X. Shen, Y.W. Zhang, Chin. J. Org. Chem. 23 (2003) 566-569; (c) M.T. Allingham, A. Howard-Jones, P.J. Murphy, D.A. Thomas, P.W.R. Caulkett, Tetrahedron Lett. 44 (2003) 8677-8680; (d) Y. Sohtome, Y. Hashimoto, K. Nagasawa, Adv. Synth. Catal. 347 (2005) 1643-1648; (e) Y. Sohtome, N. Takemura, T. Iguchi, Y. Hashimoto, K. Nagasawa, Synlett (2006) 144-146; (f) Y. Sohtome, Y. Hashimoto, K. Nagasawa, Eur. J. Org. Chem. (2006) 2894-2897; (g) H. Ube, M. Terada, Bioorg. Med. Chem. Lett. 19 (2009) 3895-3898; (h) D. Leow, C.H. Tan, Chem. -Asian J. 4 (2009) 488-507. |
| [6] |
E.J. Corey, F.Y. Zhang, Angew. Chem. Int. Ed. 38 (1999) 1931-1934. DOI:10.1002/(SICI)1521-3773(19990712)38:13/143.0.CO;2-4 |
| [7] |
T. Ooi, K. Doda, K. Maruoka, J. Am. Chem. Soc. 125 (2003) 2054-2055. DOI:10.1002/chin.200323032 |
| [8] |
(a) T. Marcelli, R.N.S. van der Hass, J.H. van Maarseveen, H. Hiemstra, Synlett (2005) 2817-2819; (b) T. Marcelli, R.N.S. van der Hass, J.H. van Maarseveen, H. Hiemstra, Angew. Chem. Int. Ed. 45 (2006) 929-931; (c) P.K. Vijaya, S.M. Murugesan, A. Siva, Org. Biomol. Chem. 14 (2016) 10101-10109; (d) M. Liu, N. Ji, L. Wang, P. Liu, W. He, Tetrahedron Lett. 59 (2018) 999-1004; (e) J. Otevrel, P. Bobal, J. Org. Chem. 82 (2017) 8342-8358; (f) Y. Sohtome, N. Takemura, K. Takada, et al., Chem. -Asian J. 2 (2007) 1150-1160; (g) X. Liu, J. Jiang, M. Shi, Tetrahedron: Asymmetry 18 (2007) 2773-2781; (h) T. Okino, S. Nakamura, T. Furukawa, Y. Takemoto, Org. Lett. 6 (2004) 625-627; (i) T.P. Yoon, E.N. Jacobsen, Angew. Chem. Int. Ed. 44 (2005) 466-468. |
| [9] |
(a) S. Gao, G. Xi, N. Li, C. Wang, J. Ma, Chin. J. Org. Chem. 30 (2010) 1811-1819; (b) Y. Alvarez-Casao, E. Marques-Lopez, R.P. Herrera, Symmetry 3 (2011) 220-245. |
| [10] |
H. Li, B. Wang, L. Deng, J. Am. Chem. Soc. 128 (2006) 732-733. DOI:10.1021/ja057237l |
| [11] |
(a) X. Chen, J. Wang, Y. Zhu, et al., Chem. -Eur. J. 14 (2008) 10896-10899; (b) T. Mandal, S. Samanta, C.G. Zhao, Org. Lett. 9 (2007) 943-945. |
| [12] |
(a) M. Vlatkovi c, L. Bernardi, E. Otten, B.L. Feringa, Chem. Commun. 50 (2014) 7773-7775; (b) M. Bandini, R. Sinisi, A. Umani-Ronchi, Chem. Commun. (2008) 4360-4362; (c) C. Palacio, S.J. Connon, Org. Lett. 13 (2011) 1298-1301. |
| [13] |
(a) M. Chennapuram, U.V.S. Reddy, C. Seki, et al., Eur. J. Org. Chem. (2017) 1638-1646; (b) M.Q. Li, J.X. Zhang, X.F. Huang, et al., Eur. J. Org. Chem. (2011) 5237-5241; (c) L. Liu, S. Zhang, F. Xue, et al., Chem. Eur. J. 17 (2011) 7791-7795; (d) P.S. Prathima, K. Srinivas, K. Balaswamy, et al., Tetrahedron: Asymmetry 22 (2011) 2099-2103. |
| [14] |
M.L. Zhang, D.F. Yue, Z.H. Wang, et al., Beilstein J. Org. Chem. 12 (2016) 295-300. DOI:10.3762/bjoc.12.31 |
| [15] |
(a) T. Shibata, N. Toshida, M. Yamasaki, S. Maekawa, K. Takagi, Tetrahedron 61 (2005) 9974-9979; (b) L.J. Deng, J. Liu, J.Q. Huang, et al., Synthesis (2007) 2565-2570; (c) K.H. Park, S.U. Song, Y.K. Chung, Tetrahedron Lett. 44 (2003) 2827-2830; (d) D.E. Kim, I.S. Kim, V. Ratovelomanana-Vidal, J.P. Genêt, N. Jeong, J. Org. Chem. 73 (2008) 7985-7989. |
| [16] |
(a) K. Yuan, S. Wang, Org. Lett. 19 (2017) 1462-1465; (b) M. Ueda, S. Sugita, A. Sato, T. Miyoshi, O. Miyata, J. Org. Chem. 77 (2012) 9344-9351. |
| [17] |
(a) M. Ueda, Y. Ikeda, A. Sato, et al., Tetrahedron 67 (2011) 4612-4615; (b) D. Sinha, A. Biswas, V.K. Singh, Org. Lett. 17 (2015) 3302-3305; (c) X. Wu, B. Wang, Y. Zhou, H. Liu, Org. Lett. 19 (2017) 1294-1297. |
| [18] |
S.A. Moteki, J. Han, S. Arimitsu, et al., Angew. Chem. Int. Ed. 51 (2012) 1187-1190. DOI:10.1002/anie.201107239 |
| [19] |
(a) J. Han, Y. Zhang, X.Y. Wu, H.N.C. Wong, Chem. Commun. 55 (2019) 397-400; (b) D. Uraguchi, K. Yamada, T. Ooi, Angew. Chem. Int. Ed. 54 (2015) 9954-9957; (c) D. Uraguchi, K. Koshimoto, T. Ooi, J. Am. Chem. Soc. 130 (2008) 10878-10879; (d) D. Uraguchi, T. Ito, S. Nakamura, T. Ooi, Chem. Sci. 1 (2010) 488-490; (e) L. Simon', R.S. Paton, J. Org. Chem. 82 (2017) 3855-3863; (f) M.A. Horwitz, B.P. Zavesky, J.I. Martinez-Alvarado, J.S. Johnson, Org. Lett. 18 (2016) 36-39; (g) T. Takeda, A. Kondoh, M. Terada, Angew. Chem. Int. Ed. 55 (2016) 4734-4737; (h) J. Yang, A.J.M. Farley, D.J. Dixon, Chem. Sci. 8 (2017) 606-610; (i) D. Uraguchi, R. Shibazaki, N. Tanaka, et al., Angew. Chem. Int. Ed. 57 (2018) 4732-4736. |
| [20] |
S. Wu, J. Tang, J. Han, D. Mao, X. Liu, X. Gao, J. Yu, L. Wang, Tetrahedron 70 (2014) 5986-5992. DOI:10.1016/j.tet.2014.05.085 |
| [21] |
(a) X. Gao, J. Han, L. Wang, Org. Lett. 17 (2015) 4596-4599; (b) X. Gao, J. Han, L. Wang, Org. Chem. Front. 3 (2016) 656-660; (c) X. Gao, J. Han, L. Wang, Synthesis 48 (2016) 2603-2611; (d) B. Li, Z.J. Xu, J. Han, Tetrahedron Lett. 59 (2018) 2412-2417. |
 2019, Vol. 30
2019, Vol. 30 


