b Department of Chemistry, Innovative Drug Research Center, Shanghai University, Shanghai 200444, China;
c Shanghai-Hong Kong Joint Laboratory in Chemical Synthesis, SIOC, CAS, Shanghai 200032, China
Substituted tetrahydroquinolines have attracted considerable attention because they display a wide range of physiological activities [1]. For their construction, palladium-catalyzed asym-metric decarboxylative [4 + 2] cycloaddition (ADC) reaction of vinyl benzoxazinanones and electron-deficient alkenes is one of powerful strategies [2-6]. In 2008, the first palladium-catalyzed ADC reaction of vinyl benzoxazinanones, using benzylidene malononitriles as electron-deficient alkenes, was reported by Tunge and co-workers using the Pd/Trost ligand catalyst system [2]. Subseq, Xiao et al. adopted activated alkenes with different electron-withdrawing groups as the substrate to produce tetrahydroquinolines with multiple contiguous stereocenters in good yields and enantioselectivities [3]. Except highly active alkenes with double activated Michael acceptors, mono-activated alkenes as reactants have also been developed. Jørgensen and Glorius groups independently reported the asymmetric [4 + 2] and [4 + 3] cycloadditions of vinyl benzoxazinanones with α, β-unsaturated aldehydes based on synergistic catalysis [4]. Very recently, Mei and Shi disclosed a catalytic asymmetric [4 + 2] cycloaddition between vinyl benzoxazinanones and methyleneindolinones to prepare diverse chiral tetrahydroquinoline-based 3, 30-pyrrolinyl spirooxindoles [5]. Although electron-deficient alkenes, which have double activators or single activator, have been reported to realize the asymmetric cyclizations, some new types of Michael acceptors are expected to extend this methodology.
3-Nitroindoles could be considered as electron-deficient alkenes [7] and have been successfully used in the Pd-catalyzed [3 + 2] cycloadditions [8] through the dearomatization approach [9]. As part of our ongoing efforts to Pd-catalyzed asymmetric cycloaddition reaction [10], we speculated electron-deficient 3-nitroindoles could be used for asymmetric [4 + 2] cycloaddition with vinyl benzoxazinanone to deliver the corresponding tetrahydro-5H-indolo[2, 3-b]quinolines, which exist as key structural motifs found in an array of bioactive natural products (Fig. 1) [11].

|
Download:
|
| Fig. 1. Representative biologically active natural products. | |
Efforts were initially focused on the reaction between 3-nitroindole 1a with vinyl benzoxazinanone 2a in the presence of catalytic amount of Pd2(dba)3CHCl3 (Table 1). When using PPh3 as the ligand, no cycloaddition product was afforded (entry 1). Ligand L1, an efficient ligand in the Pd-catalyzed asymmetric [3 + 2] cycloaddition of vinylaziridine with 3-nitroindoles [10d], resulted in the formation of the desired product 3a in 31% yield with >95:5 dr and 30% ee (entry 2). Yield of 3a increased to 57% when (R)-BINAP was used as the ligand, but the enantioselectivity was poor (entry 3). Pleasingly, the ee value of product 3a was increased to 72% with the yield of 59% by using SIOCPhox L2 developed by our group as the ligand (entry 4) [12]. A thorough screening of different SIOCPhox ligands was then investigated further (entries 4–11). Product 3a was afford with 91% ee in the case of L8 being the ligand, but the yield is low (entry 10). Examination of solvent effect on the reaction (entries 12–18) revealed that the reaction in dioxane provided better enantioselectivity, though the yield was still not satisfied (entry 13 vs. 10). Increasing the catalyst loading did not improve the yield of the reaction (entry 19 vs. 13). To further improve the yield of the cycloaddition, the impact of the N-protecting groups of 3-nitroindole was investigated. To our delighted, the yield of the cycloaddition was increased to 86% when N-Ts-3-introindole 1d was used with an excellent enantioselectivity of 93% (entry 20). A further increase of yield to 97% was observed by changing the N-protecting group to Ns, but the ee value decreased to 85% (entry 21). N-Ac-3-nitroindole and N-Boc-3-nitroindole were tested, but no reaction took place (data not shown). The influence of the N-protecting group on the vinyl benzoxazinanone was also studied. When tosyl (Ts) group on the 2a was replaced with proton, nosyl, or acetyl, the cycloaddition did not occur (data not shown).
|
|
Table 1 Optimization of the Pd-catalyzed [4 + 2] cycloaddition of 3-nitroindole 1 with vinyl benzoxazinanone 2a.a |
Under optimized conditions, we examined the substrate scope of the Pd-catalyzed asymmetric [4 + 2] cycloaddition (Table 2). First, various substituted N-Ts-3-nitroindoles 1 were examined by reacting with vinyl benzoxazinanone 2a. A range of 4-, 5-, 6-, 7-substituents with different electronic properties were well tolerated to produce the corresponding products 3 with uniformly excellent diastereoselectivity and enantioselectivity (>95:5 dr, 92%–95% ee) (entries 2, 4–8). When a Chlorine atom is introduced to the C-4 position, ee value of the product 3e was reduced to 79% (entry 3). When a methoxycarbonyl group is placed at the C-5 position of N-CO2Me-3-nitroindole 1m, the corresponding adduct 3p was obtained in 91% yield with 95% ee (entry 14). Attention was then turned to the variation in substituents on the benzoxazinanone 2. Reaction of 2 with electron-donating group (MeO, Me) and halogen substituent (Br) at the 6-, 7- and 8- positions gave the corresponding tetrahydro-5H-indolo[2, 3-b]quinolines 3 in 86%– 90% yield, 87%–96% ee with >95:5 dr (entries 9–13). Vinyl benzoxazinanone 2e reacted with N-CO2Me-(1a) and N-Boc-3- nitroindole (1n and 1o) also well in this cycloaddition to afford corresponding cycloadducts in high yields with excellent dr and ee (entries 15–17). Unfortunately, indole-3-carbonitrile, 3-benzoylindole proved to be unreactive in the cycloaddition, while no reaction occurred by using 3-tosyl-6-vinyl-1, 3-oxazinan-2-one as the reactant (data not shown).
|
|
Table 2 Substrate scope of the palladium-catalyzed asymmetric [4 + 2] cycloaddition of 3-nitroindoles 1 with vinyl benzoxazinanones 2.a |
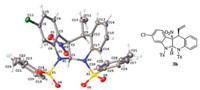
The absolute configuration of product 3h was determined unambiguously as (5S, 10S, 11S) via single-crystal X-ray diffraction analysis (CCDC 1905027) (Fig. 2). For its structural details, see the Supporting information.
|
Download:
|
| Fig. 2. ORTEP drawing of product 3h. | |
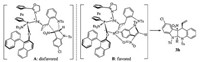
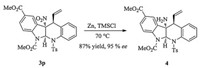
To account the diastereo- and enantioselectivities of the reaction, a plausible transition state was proposed on the basis of the absolute configuration of product 3h and literature works [3, 10b, 12j]. As depicted in Fig. 3, the intermediate B is favored over the intermediate A, because of the presence of a hydrogen bond between the hydroxy group of the ligand and the nitro group of the 3-nitroindole. Chemical transformation of the indoline product is also demonstrated (Scheme 1). The nitro group on the quaternary carbon center was reduced to a free amine 4 using zinc powder and TMSCl.
|
Download:
|
| Fig. 3. Proposed transition states of the reaction. | |
|
Download:
|
| Scheme 1. Transformation of product 3p. | |
In conclusion, we have established a Pd-catalyzed asymmetric decarboxylative [4 + 2] cycloaddition of 3-nitroindoles and vinyl benzoxazinanones. This reaction provides an efficient protocol for constructing a series of chiral tetrahydro-5H-indolo[2, 3-b] quinolines in high yields and with excellent diastereo- and enantioselectivities. Research into further applications of this enantioselective [4 + 2] cycloaddition is in progress.
AcknowledgmentsWe acknowledge financial support from the National Natural Science Foundation of China (NSFC, Nos. 21772215, 21532010 and 21472214), the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20030100), the Chinese Academy of Sciences, the Technology Commission of Shanghai Municipality, and the Croucher Foundation of Hong Kong.
Appendix A. Supplementary dataSupplementarymaterial related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.028.
| [1] |
(a) B.S. Rauckman, M.Y. Tidwell, J.V. Johnson, B. Roth, J. Med. Chem. 32 (1989) 1927-1935; (b) A.R. Katritzky, S. Rachwal, B. Rachwal, Tetrahedron 52 (1996) 15031-15070; (c) D.H. Barton, K. Nakanishi, O.M. Cohn, Comprehensive Natural Products Chemistry, Elsevier, Oxford, 1999; (d) V.P. Sridharan, A. Suryavanshi, J.C. Menéndez, Chem. Rev. 111 (2011) 7157-7259. |
| [2] |
C. Wang, J.A. Tunge, J. Am. Chem. Soc. 130 (2008) 8118-8119. DOI:10.1021/ja801742h |
| [3] |
Y. Wei, L.Q. Lu, T.R. Li, et al., Angew. Chem. Int. Ed. 55 (2016) 2200-2204. DOI:10.1002/anie.201509731 |
| [4] |
(a) L.A. Leth, F. Glaus, M. Meazza, et al., Angew. Chem. Int. Ed. 55 (2016) 15272-15276; (b) C. Guo, M. Fleige, D. Janssen-Müller, C.G. Daniliuc, F. Glorius, J. Am. Chem. Soc. 138 (2016) 7840-7843; (c) C. Guo, D. Janssen-Müller, M. Fleige, et al., J. Am. Chem. Soc. 139 (2017) 4443-4451. |
| [5] |
G.J. Mei, D. Li, G.X. Zhou, et al., Chem. Commun. 53 (2017) 10030-10033. DOI:10.1039/C7CC05595A |
| [6] |
(a) T.R. Li, Y.N. Wang, W.J. Xiao, L.Q. Lu, Tetrahedron Lett. 59 (2018) 1521-1530; (b) N. De, E.J. Yoo, ACS Catal. 8 (2018) 48-58; (c) G.J. Mei, C.Y. Bian, G.H. Li, et al., Org. Lett. 19 (2017) 3219-3222; (d) M.M. Li, Y. Wei, J. Liu, et al., J. Am. Chem. Soc. 139 (2017) 14707-14713; (e) Y.N. Wang, B.C. Wang, M.M. Zhang, et al., Org. Lett. 19 (2017) 4094-4097; (f) H.W. Zhao, N.N. Feng, J.M. Guo, et al., J. Org. Chem. 83 (2018) 9291-9299; (g) J.H. Jin, H. Wang, Z.T. Yang, et al., Org. Lett. 20 (2018) 104-107; (h) C. Wang, Y. Li, Y. Wu, et al., Org. Lett. 20 (2018) 2880-2883; (i) S. Duan, B. Cheng, X. Duan, et al., Org. Lett. 20 (2018) 1417-1420; (j) N. Punna, P. Das, V. Gouverneur, N. Shibata, Org. Lett. 20 (2018) 1526-1529; (k) Y.N. Lu, J.P. Lan, Y.J. Mao, et al., Chem. Commun. 54 (2018) 13527-13530; (l) M. Sun, X. Wan, S.J. Zhou, G.J. Mei, F. Shi, Chem. Commun. 55 (2019) 1283-1286. |
| [7] |
(a) E.T. Pelkey, L. Changa, G.W. Gribble, Chem. Commun. (1996) 1909-1910; (b) A. Awata, T. Arai, Angew. Chem. Int. Ed. 53 (2014) 10462-10465; (c) B.M. Trost, V. Ehmke, B.M. O'Keefe, D.A. Bringley, J. Am. Chem. Soc. 136 (2014) 8213-8216; (d) J.Q. Zhao, Z.J. Wu, M.Q. Zhou, et al., Org. Lett. 17 (2015) 5020-5023; (e) A.L. Gerten, L.M. Stanley, Org. Chem. Front. 3 (2016) 339-343; (f) Y. Li, F. Tur, R.P. Nielsen, et al., Angew. Chem. Int. Ed. 55 (2016) 1020-1024; (g) X.H. Liu, D.X. Yang, K.Z. Wang, J.L. Zhang, R. Wang, Green Chem. 19 (2017) 82-87. |
| [8] |
(a) D.J. Rivinoja, Y.S. Gee, M.G. Gardiner, J.H. Ryan, C.J.T. Hyland, ACS Catal. 7 (2017) 1053-1056; (b) M. Laugeois, J. Ling, C. Fe'rard, et al., Org. Lett. 19 (2017) 2266-2269; (c) Y.S. Gee, D.J. Rivinoja, S.M. Wales, et al., J. Org. Chem. 82 (2017) 13517-13529; (d) J.Q. Zhang, F.F. Tong, B.B. Sun, et al., J. Org. Chem. 83 (2018) 2882-2891; (e) Q. Cheng, F. Zhang, Y. Cai, Y.L. Guo, S.L. You, Angew. Chem. Int. Ed. 57 (2018) 2134-2138; (f) M. Sun, Z.Q. Zhu, L. Gu, et al., J. Org. Chem. 83 (2018) 2341-2348. |
| [9] |
(a) C.X. Zhuo, W. Zhang, S.L. You, Angew. Chem. Int. Ed. 51 (2012) 12662-12686; (b) C.X. Zhuo, C. Zheng, S.L. You, Acc. Chem. Res. 47 (2014) 2558-2573; (c) C. Zheng, S.L. You, Chem 1 (2016) 830-857; (d) W.T. Wu, L. Zhang, S.L. You, Chem. Soc. Rev. 45 (2016) 1570-1580. |
| [10] |
(a) W.Q. Wu, C.H. Ding, X.L. Hou, Synlett 23 (2012) 1035-1038; (b) C.F. Xu, B.H. Zheng, J.J. Suo, C.H. Ding, X.L. Hou, Angew. Chem. Int. Ed. 54 (2015) 1604-1607; (c) J.J. Suo, J. Du, Q.R. Liu, et al., Org. Lett. 19 (2017) 6658-6661; (d) J.J. Suo, W. Liu, J. Du, C.H. Ding, X.L. Hou, Chem.-Asian J. 13 (2018) 959-963; (e) W.Y. Wang, J.Y. Wu, Q.R. Liu, et al., Org. Lett. 20 (2018) 4773-4776; (f) J. Du, Y.J. Jiang, J.J. Suo, et al., Chem. Commun. 54 (2018) 13143-13146. |
| [11] |
(a) A. Numata, C. Takahashi, Y. Ito, et al., Tetrahedron Lett. 34 (1993) 2355-2358; (b) R. Jadulco, R.A. Edrada, R. Ebel, et al., J. Nat. Prod. 67 (2004) 78-81; (c) P.W.Dalsgaard, J.W. Blunt, M.H.G.Munro, J.C. Frisvad, C.Christophersen, J.Nat. Prod. 68 (2005) 258-261. |
| [12] |
(a) S.L. You, X.Z. Zhu, Y.M. Luo, X.L. Hou, L.X. Dai, J. Am. Chem. Soc. 123 (2001) 7471-7142; (b) W.H. Zheng, N. Sun, X.L. Hou, Org. Lett. 7 (2005) 5151-5154; (c) X.X. Yan, C.G. Liang, Y. Zhang, et al., Angew. Chem. Int. Ed. 44 (2005) 6544-6546; (d) W.H. Zheng, B.H. Zheng, Y. Zhang, X.L. Hou, J. Am. Chem. Soc. 129 (2007) 7718-7719; (e) K. Zhang, Q. Peng, X.L. Hou, Y.D. Wu, Angew. Chem. Int. Ed. 47 (2008) 1741-1744; (f) J.P. Chen, C.H. Ding, W. Liu, X.L. Hou, L.X. Dai, J. Am. Chem. Soc. 132 (2010) 15493-15495; (g) B.H. Zheng, C.H. Ding, X.L. Hou, Synlett 2011 (2011) 2262-2264; (h) T.G. Chen, P. Fang, L.X. Dai, X.L. Hou, Synthesis 47 (2015) 134-140; (i) X.H. Li, S.L. Wan, D. Chen, et al., Synthesis 48 (2016) 1568-1572; (j) J.Q. Huang, W. Liu, B.H. Zheng, et al., ACS Catal. 8 (2018) 1964-1972. |
 2019, Vol. 30
2019, Vol. 30