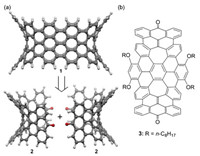
Negatively curved carbon allotropes are theoretical structures of sp2 carbon atoms, which present saddle-shaped surfaces as a result of embedding seven- or eight-membered rings in a graphitic network. Negatively curved periodical structures of carbons are known as Mackay crystals or carbon schwarzites because Mackay together with Terrones in 1991 first proposed negatively curved carbon allotropes [1], whose topological model was described by Schwarz, a German mathematician [2]. Although they are predicted to have interesting properties and potential applications on the basis of computational studies [3-5], negatively curved carbon allotropes are yet to be synthesized. A promising bottomup approach to negatively curved nanocarbon materials is synthesis of negatively curved nanographenes, which are saddle-shaped polycyclic arenes containing seven- or eight-mem-bered rings [6]. They are not only segments of negatively curved carbon allotropes containing important structural information, but also are envisioned as templates or monomer units for synthesis of negatively curved carbon allotropes in a controlled growth process or by polymerization, respectively [7, 8]. As inspired by this idea, a few negatively curved nanographenes have been designed and synthesized by us [9, 10] and other research groups [11] since 2012. A further step toward negatively curved carbon allotropes is synthesis of negatively curved carbon nanobelts or nanotubes, which present key segments of Mackay crystals. We envision that a negatively curved carbon nanobelt can in principle be synthesized by connecting properly functionalized aromatic saddles, whose curved structure can facilitate formation of a macrocycle. For example, as shown in Fig. 1a, nanobelt 1 can in principle be synthesized by connecting two molecules of ketone-functionalized aromatic saddle (2) through a McMurry reaction and subsequent oxidative cyclodehydrogenation or photo-chemical cyclization. Herein, we report synthesis and crystal structure of 3 (Fig. 1b), which is an alkoxylated derivative of 2.
|
Download:
|
| Fig. 1. (a) Retrosynthesis of negatively curved carbon nanobelt 1; (b) structure of ketone-functionalized aromatic saddle 3. | |
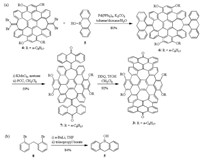
As shown in Scheme 1a, the synthesis of 3 started from the Suzuki coupling of compound 4 and borinic acid 5, which gave compound 6 in a good yield (80%). Compound 4 was reported by us earlier [10], while borinic acid 5 is a new compound, which was synthesized for the first time in this study. As shown in Scheme 1b, halogen-lithium exchange of 8 [12] with n-butyllithium followed by quenching with 1.3 equiv. of triisopropyl borate yielded borinic acid 5 as the major product in a yield of 84%. The structure of 5 was unambiguously identified by X-ray crystallography (CCDC No. 1893914 contains the crystallographic data of 5) as shown in Fig. 2a. It is worth mentioning that the Suzuki coupling of diarylborinic acid is rare in the literature [13], and using such a reaction to form an extra ring [14] is even rarer. The two methylene bridges in 6 were converted to carbonyl groups in 7 by successive oxidation using potassium permanganate and pyridinium chlorochromate. When potassium permanganate was used alone, the corresponding diol was identified by 1H NMR but could not be separated by column chromatography from the reaction mixture in a pure form. Finally, the Scholl reaction of 7 with DDQ and triflic acid gave compound 3 in excellent yield (92%). However, our attempts to connect two molecules of 3 into a macrocycle through McMurry coupling reaction or Barton-Kellogg reaction were not successful.
|
Download:
|
| Scheme 1. (a) Synthesis of 3 and (b) preparation of 5. | |
|
Download:
|
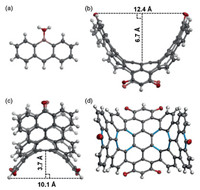
| Fig. 2. Structures of 5 and 3 in single crystals: (a) top view of 5; (b) side view of 3; (c) front view of 3; (d) top view of 3. (Carbon, oxygen and boron atoms are shown with ellipsoids set at 50% probability, and octyl groups in 3 are removed for clarity.). | |
Compound 3 is soluble in common organic solvents resulting in red solutions and appears almost nonfluorescent. As shown in Fig. S1 in Supporting information, the UV–vis absorption of 3(5×10-6 mol/L in CH2Cl2) exhibits a broad and relatively weak absorption band in the visible light region with λmax of 495 nm. The cyclic voltammogram of 3 in CH2Cl2 (Fig. S2 in Supporting information) exhibits one reversible oxidation wave with a halfwave oxidation potential of 0.42 V vs. ferrocenium/ferrocene (Fc+/Fc) and one reversible reduction wave with a half-wave reduction potential of -1.68 V vs. Fc+/Fc. From the half-wave oxidation and reduction potentials, the HOMO and LUMO energy level of 3 are estimated as -5.52 eV and -3.42 eV, respectively [15], which lead to a HOMO-LUMO gap of 2.1 eV. It is in good agreement with the optical gap of 2.07 eV as obtained from the absorption edge at around 600 nm.
Crystals of 3 were characterized with X-ray crystallography (CCDC No. 1893913 contains the crystallographic data of 3). As shown in Figs. 2b and c, the polycyclic backbone of 3 is curved like a saddle, which is 12.4 Å wide and 6.7 Å deep in the upper part, and is 10.1 Å wide and 3.7 Å deep in the lower part. As a result, 3 presents a deeper saddle shape than closely related aromatic saddles reported by us earlier [10], and resembles half of a negatively curved carbon nanobelt. Having a bond length of 1.46–1.48 Å, the C—C bonds shown in blue are significantly longer than a typical C—C aromatic bond (1.38–1.40 Å) but resemble C—C single bonds between sp2-sp2 carbons, which have a typical bond length of 1.45–1.48 Å depending on the degree of conjugation [16]. This indicates that the π-bonds are not fully delocalized in the polycyclic backbone. Instead, the p-bonds are largely localized on the aromatic sextets according to Clar's rule. [17].
In summary, a novel ketone-functionalized aromatic saddle (3) was successfully synthesized and unambiguously identified with X-ray crystallography. It can, in principle, be used as a building block for synthesis of negatively curved carbon nanobelts, although our preliminary attempts to connect two molecules of 3 into a macrocycle through reactions of carbonyl groups were not successful.
AcknowledgmentsWe thank Ms. Hoi Shan Chan (the Chinese University of Hong Kong) for the single crystal crystallography. This work was supported by the Research Grants Council of Hong Kong (No. GRF 14300218). This work is dedicated to Prof. Henry N. C. Wong.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.013.
| [1] |
A.L. Mackay, H. Terrones, Nature 352 (1991) 762-762. |
| [2] |
R. Vajtai, Springer Handbook of Nanomaterials, Springer, Berlin Heidelberg, Berlin, Heidelberg, 2013, pp. 83-104.
|
| [3] |
S. Park, K. Kittimanapun, J.S. Ahn, Y.K. Kwon, D. Tománek, J. Phys. Condens. Matter. 22 (2010) 334220.
|
| [4] |
N. Park, M. Yoon, S. Berber, et al., Phys. Rev. Lett. 91(2003) 237204.
|
| [5] |
D. Odkhuu, D.H. Jung, H. Lee, et al., Carbon 66 (2014) 39-47. DOI:10.1016/j.carbon.2013.08.033 |
| [6] |
S.H. Pun, Q. Miao, Acc. Chem. Res. 51 (2018) 1630-1642. DOI:10.1021/acs.accounts.8b00140 |
| [7] |
Y. Segawa, H. Ito, K. Itami, Nat. Rev. Mater. 1(2016) 15002.
|
| [8] |
A. Narita, X.Y. Wang, X. Feng, K. Müllen, Chem. Soc. Rev. 44 (2015) 6616-6643. DOI:10.1039/C5CS00183H |
| [9] |
(a) J. Luo, X. Xu, R. Mao, Q. Miao, J. Am. Chem. Soc. 134 (2012) 13796-13803; (b) X. Yang, Q. Miao, Synlett 27 (2016) 2091-2094; (c) X. Gu, H. Li, B. Shan, Z. Liu, Q. Miao, Org. Lett. 19 (2017) 2246-2249; (d) K.Y. Cheung, C.K. Chan, Z. Liu, Q. Miao, Angew. Chem. Int. Ed. 56 (2017) 9003-9007; (e) S.H. Pun, C.K. Chan, J. Luo, Z. Liu, Q. Miao, Angew. Chem. Int. Ed. 57 (2018) 1581-1586. |
| [10] |
K.Y. Cheung, X. Xu, Q. Miao, J. Am. Chem. Soc. 137 (2015) 3910-3914.
|
| [11] |
(a) K. Kawasumi, Q. Zhang, Y. Segawa, L.T. Scott, K. Itami, Nat. Chem. 5 (2013) 739-744; (b) C.N. Feng, M.Y. Kuo, Y.T. Wu, Angew. Chem. Int. Ed. 52 (2013) 7791-7794; (c) Y. Sakamoto, T. Suzuki, J. Am. Chem. Soc. 135 (2013) 14074-14077; (d) R.W. Miller, A.K. Duncan, S.T. Schneebeli, D.L. Gray, A.C. Whalley, Chem. -Eur. J. 20 (2014) 3705-3711; (e) R.W. Miller, S.E. Averill, S.J. Van Wyck, A.C. Whalley, J. Org. Chem. 81 (2016) 12001-12005; (f) I.R. Márquez, N. Fuentes, C.M. Cruz, et al., Chem. Sci. 8 (2017) 1068-1074. |
| [12] |
T.K. Wood, W.E. Piers, B.A. Keay, M. Parvez, Chem. -Eur. J. 16 (2010) 12199-12206.
|
| [13] |
(a) D.D. Winkle, K.M. Schaab, Org. Process Res. Dev. 5 (2001) 450-451; (b) X. Chen, H. Ke, Y. Chen, C. Guan, G. Zou, J. Org. Chem. 77 (2012) 7572-7578; (c) H. Ke, X. Chen, G. Zou, J. Org. Chem. 79 (2014) 7132-7140. |
| [14] |
E. Dimitrijevi c, M. Cusimano, M.S. Taylor, Org. Biomol. Chem. 12(2014) 1391.
|
| [15] |
C.M. Cardona, W. Li, A.E. Kaifer, D. Stockdale, G.C. Bazan, Adv. Mater. 23 (2011) 2367-2371. DOI:10.1002/adma.201004554 |
| [16] |
E.V. Anslyn, D.A. Dougherty, Modern Physical Organic Chemistry, University Science, Sausalito, CA, 2004 p. 22.
|
| [17] |
E. Clar, The Aromatic Sextet, J. Wiley, London, New York, 1972.
|
 2019, Vol. 30
2019, Vol. 30 

