Transition metal-catalyzed, especially palladium-catalyzed coupling reactions have emerged as widely applicable methods for synthesizing structurally diverse organic compounds via formation of carbon-carbon bonds [1]. Although palladiumcatalyzed cross-coupling between alkenyl halides and organolithium compounds was initially reported in 1970s [2, 3], a direct use of organolithium reagents in cross-coupling reactions had been neglected for a long time, mainly due to the limitations of organolithium reagents such as their high reactivities and low selectivities. Recently, Feringa and co-workers developed palladium-based catalytic systems to form diverse carbon-carbon bonds using organolithium compounds as cross-coupling partners [4-12]. While palladium-based catalysts typically mediated such reactions, there are increasing concerns about its high cost, low natural abundance, environmentally deleterious extraction and toxicity. Therefore, there is a growing interest in replacing palladium-based catalysts with iron-based catalysts because iron is a more earth-abundant and environmentally friendly element [13]. In this account, we summarized our recent progress on ironcatalyzed coupling reaction involving organolithium reagents (Fig. 1).

|
Download:
|
| Fig. 1. Transition metal-catalyzed cross-coupling to form carbon-carbon bonds. | |
2. C(sp2)-C(sp3) cross-coupling of halides with alkyllithium reagents 2.1. C(sp2)-C(sp3) cross-coupling of aryl halides with alkyllithium reagents
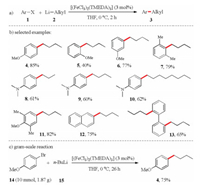
After detecting iron-catalyzed cross-coupling product in the synthetic studies toward tetrabenzo[a, c, e, g]cyclooctatetraene (tetraphenylene) derivatives, we recognized the potential use of lithium reagents in iron-catalyzed cross-coupling reactions [14]. We initially focused on iron-catalyzed cross-coupling between aryl halides and alkyllithium reagents (Scheme 1). We examined a series of catalysts, ligands, solvents and temperatures, then the use of iron complex [(FeCl3)2(TMEDA)3] (3 mol%) in THF at 0 ℃ was chosen as the best reaction condition. As shown in Scheme 1, electron-donating groups and bulky functional groups facilitated the cross-coupling reaction without sacrificing the yields of the corresponding products. In addition, a series of freshly prepared alkyllithiums were also compatible with this protocol. As the capability of scalable production for laboratory and industry usage is emerging as a very essential goal in chemical reactions, we also confirmed the scalable feasibility of iron-catalyzed reactions. As shown in Scheme 1, iron-catalyzed cross-coupling of 4-bromoanisole (14) and n-butyllithium (15) on gram-scale provided the relevant product in satisfactory yield (Scheme 1c).
|
Download:
|
| Scheme 1. Iron-catalyzed cross-coupling of aryl halides with alkyllithium reagents. | |
To our surprise, when isopropyllithium (17), a typical secondary organolithium, was utilized in the iron-catalyzed cross-coupling system with 4-bromoanisole (14), 1-isopentyl-4-methoxybenzene (19) was obtained together with a trace amount of directly crosscoupling product, namely 1-isopropyl-4-methoxybenzene [14]. After prolonging the reaction time to overnight at 22 ℃, the yield of 1-isopentyl-4-methoxybenzene was optimized up to 71% (Scheme 2). Several aryl bromides were also investigated to explore the substituent effect at various positions of the benzene ring. Moreover, treatment of 4-bromoanisole (14) with isopropyllithium (17) in THF-d8 led to the formation of the deuterated product 24 in 61% yield (Scheme 2). This is an unusual example of transition metal-catalyzed cross-coupling reaction involving freshly prepared ethylene generated by decomposing THF with isopropyllithium. This reaction also worked well on gram-scale (Scheme 2c).

|
Download:
|
| Scheme 2. Iron-catalyzed release-capture ethylene coupling with isopropyllithium reagents. | |
2.2. C(sp2)-C(sp3) cross-coupling of alkenyl iodides with alkyllithium reagents
Due to the application of substituted alkenes in a broad range of chemical transformations, we uncovered an efficient iron-catalyzed cross-coupling of alkenyl iodides with alkyllithium reagents [15]. As shown in Scheme 3, the best condition was found to be the use of Fe(acac)2 (5 mol%) and DavePhos (5 mol%) in toluene at 23 ℃. Reaction results on different alkyllithium reagents showed that primary lithium reagents were well compatible with good yields, and secondary alkyl lithium reagents were also compatible with slightly inferior yields. To our delight, both electronwithdrawing groups and electron-donating groups can be well tolerated on the substrates. It is noteworthy that only a very little percentage of isomerization was found in Z-alkenyl iodide systems (Scheme 3, 36-38), which supports our hypothesis that this reaction might likely not undergo a radical pathway. We also confirmed the scalable feasibility of this iron-catalyzed reaction, which in gram-scale provided desired product in satisfactory yield (Scheme 3c).

|
Download:
|
| Scheme 3. Iron-catalyzed cross-coupling of alkenyl iodides with alkyllithium reagents. | |
3. C(sp3)-C(sp3) and C(sp3)-C(sp2) cross-coupling of alkyl halides with organolithium reagents
We also extended the iron catalysis strategy to alkyl halides with organolithium reagents (Scheme 4) [14]. Typically, commercially available 1-bromo-3-phenylpropane (57) was allowed to react with n-BuLi (15) to explore the possibility of C(sp3)-C(sp3) cross-coupling. Gratifyingly, the reaction proceeded smoothly and the desired product was isolated in 77% yield (67% yield on gramscale). Other organolithium reagents, such as cyclopropyllithium, 9H-fluoren-9-yllithium and (trimethylsilyl)methyllithium also coupled with 1-bromo-3-phenylpropane to provide the corresponding C(sp3)-C(sp3) cross-coupling products in good to excellent yields. The same procedure also allowed the C(sp3)- C(sp2) cross-coupling of alkyl bromide with phenyllithium reagent (Scheme 4, 56).

|
Download:
|
| Scheme 4. Iron-catalyzed cross-coupling of alkyl bromides with alkyllithium reagents or phenyllithium reagents. | |
4. C(sp2)-C(sp2) coupling of alkenyllithium reagents
Since the 1, 3-butadiene frameworks appear in many biologically active natural products and participate in a variety of useful chemical syntheses, we then turned our attention to investigate C(sp2)-C(sp2) (vinyl-vinyl) construction.
4.1. C(sp2)-C(sp2) cross-coupling of alkenyl halides with alkenyllithium reagentsOur iron-catalyzed C(sp2)-C(sp2) cross-coupling reactions began with the preliminary screening of diverse vinyl halides with (E)-propenyllithium in the presence of various commonly used iron catalysts in toluene or THF [16]. Under these conditions, iodide was found to be the best halide for this type of reactions. After optimization based on cross-coupling of (E)-β-phenyl vinyl iodide with (E)-propenyllithium, a broad range of vinyl iodides and vinyllithium reagents were also examined (Scheme 5). As expected, fluorine-, chlorine- and bromine-substituted phenyl vinyl iodides successfully underwent the cross-coupling with excellent chemo-selectivity to give the products in moderate to good yields. We were glad to find out that both electronwithdrawing group substituted vinyl iodides and electrondonating group substituted vinyl iodides successfully underwent this cross-coupling reaction to afford the corresponding 1, 3-dienes in acceptable yields. Furthermore, typical gram-scale reaction smoothly provided the desired 1, 3-butadiene 73 in satisfactory yield (Scheme 5c).

|
Download:
|
| Scheme 5. Iron-catalyzed cross-coupling of alkenyl iodides with alkenyllithium reagents. | |
4.2. C(sp2)-C(sp2) homo-coupling of alkenyllithium reagents
As shown in Scheme 6, we continued to carry out iron-catalyzed C(sp2)-C(sp2) homo-coupling of alkenyllithiums to generate the relevant 1, 3-dienes [17]. After optimizing the experimental parameters, we examined a broad range of substrate scope. As anticipated, various polycyclic dienes, especially a dimer of natural product derivative 90, were obtained in acceptable to high yields. Notably, alkyl substituted diene 86 was isolated in excellent yield (98%). Furthermore, a typical gram-scale reaction was performed to afford the product in high yield (Scheme 6c).

|
Download:
|
| Scheme 6. Iron-catalyzed homo-coupling of alkenyllithium reagents. | |
Since organolithiums were employed in cyclization reactions, generating new carbon-carbon bonds [18], we next employed cyclization of acetylenic alkyllithium to in-situ generated vinyllithium species, which then underwent our oxidative homocoupling procedure to generate polycyclic all-substituted 1, 3- dienes [17]. As shown in Scheme 7, 1, 3-dienes with two 5- or 4- membered rings could be easily obtained in satisfactory yields. In contrast, butadiene 102 with two 6-membered rings was formed in low yield because of allene formation [18]. Gratifyingly, several acetylenic phenyl iodides generated butadienes with two fluorene rings (Scheme 7, 104-106), which were applied to construct the corresponding dispirocycles showing high fluorescence quantum yields in the field of optoelectronics [19]. To demonstrate the practicality of this protocol, the coupling reaction with substrate 107 was carried out on gram-scale to afford diene compound 94 in 64% yield.

|
Download:
|
| Scheme 7. Tandem anionic cyclization/iron-catalyzed homo-coupling of alkenyllithium reagents. | |
5. Mechanism studies 5.1. Mechanism studies for C(sp2)-C(sp3) cross-coupling of alkenyl iodides with alkyllithium reagents
To gain more insights into the reaction mechanism, control experiments in iron-catalyzed cross-coupling of alkenyl iodides with alkyllithium reagents were performed (Scheme 8) [15]. During the cross coupling reaction of substrate 108, 1.0 equiv. of radical scavenger TEMPO (2, 2, 6, 6-tetramethyl-1-piperidinyloxy) was added to the reaction mixture under standard conditions. It was found that the yield of 109 was dramatically decreased from 82% to 0%. Moreover, when 20% of TEMPO was added, the yield of 109 dropped to 50% rather than 0%, as compared with 100% of TEMPO. In both experiments, the TEMPO-n-Bu adduct 110 was observed by GC-MS (Scheme 8a). However, these results did not necessarily indicate that this reaction went through a radical pathway, in comparison to control experiments (Scheme 8b). It is clear that substrate 108 cannot react with TEMPO, but the other reagent, n-butyllithium could be coupled with TEMPO directly even without any iron catalysts. Combined with our previous experiments (Schemes 8a and b), there is no clear evidence to support the notion that the decreasing yield is caused by the trapped radical or the insufficient lithium reagents. Furthermore, the reaction of 111 was carried out and monitored very carefully (Scheme 8c), but only trace isomerization product 113 was observed (Z/E > 15:1). On the other hand, radical clock experiments of 114 and 116 were also performed. The results indicated that no ring-closing product or ring-opening product was observed, therefore hinting the absence of transient radical intermediates (Scheme 8d). These studies suggested that radical pathways were not likely to be involved in this reaction. Based on those experimental results, we proposed a plausible mechanism for iron-catalyzed cross-coupling of alkenyl iodides with alkyllithium reagents (Fig. 2).

|
Download:
|
| Scheme 8. Control experiments in iron-catalyzed cross-coupling of alkenyl iodides with alkyllithium reagents. | |

|
Download:
|
| Fig. 2. Proposed mechanism for iron-catalyzed cross-coupling of alkenyl iodides with alkyllithium reagents. | |
5.2. Mechanism studies for C(sp2)-C(sp2) homo-coupling of alkenyllithium reagents
In order to obtain mechanistic insights of the transformation, several control experiments were carried out [17]. One equivalent of FeCl2 also worked well, but iron powder did not work in this oxidative homo-coupling. Moreover, a catalytic amount of iron power and stoichiometric oxidant failed to initiate the catalytic cycle. Therefore, we proposed Fe(Ⅰ) as the lowest oxidation state in this homo-coupling.
Based on those experimental results, we proposed a plausible mechanism of oxidative homo-coupling of alkenyllithium reagents (Fig. 3). At the begining, catalytic amounts of FeCl3 and vinyllithium reagents generate the tetra-coordinated complex A, which readily undergoes a reductive elimination of the homo-coupling product to form Fe(Ⅰ) complex B. In the presence of DTBP, Fe(Ⅰ) complex B would be oxidized to Fe(Ⅲ) complex C. Subsequently, complex C would regenerate the reactive complex A with two more molecules of vinyllithium, thus completing the catalytic cycle. In order to understand and to support partially our proposed mechanism, DFT computation and EPR experiments on ironcatalyzed oxidative homo-coupling were performed [17].

|
Download:
|
| Fig. 3. Proposed mechanism for iron-catalyzed oxidative homo-coupling of alkenyllithium reagents. | |
6. Conclusions and outlook
In summary, we have developed several iron-catalyzed crosscoupling reactions between organohalides and organolithiums to construct diverse carbon-carbon bonds, and iron-catalyzed oxidative homo-coupling reactions of vinyllithiums to construct di-, tetra- and hexa-substituted 1, 3-butadienes. These reaction systems, involving inexpensive and environmentally friendly iron catalysts, are easy to scale up and would help to open up a new avenue to the synthesis of carbon-carbon bonds in materials. Further studies on the syntheses and applications of other types of carbon-carbon and carbon-heteroatom bonds, and mechanistic investigation are in progress in our laboratories.
AcknowledgmentsThis work was financed by National Natural Science Foundation of China (Nos. 21672181, 21272199), GRF/RGC (Nos. 403012, CUHK14309216, CUHK14303815), grant to the State Key Laboratory of Synthetic Chemistry from the Innovation and Technology Commission, The Chinese Academy of Sciences-Croucher Foundation Funding Scheme for Joint Laboratories, and Direct Grant (No. 4053325) from The Chinese University of Hong Kong.
| [1] |
E.I. Negishi, Angew. Chem. Int. Ed. 50 (2011) 6738-6764. DOI:10.1002/anie.201101380 |
| [2] |
M. Yamamura, I. Moritani, S.I. Murahashi, J. Organomet. Chem. 91 (1975) C39-C42. DOI:10.1016/S0022-328X(00)89636-9 |
| [3] |
S. Murahashi, M. Yamamura, K. Yanagisawa, N. Mita, K. Kondo, J. Org. Chem. 44 (1979) 2408-2417. DOI:10.1021/jo01328a016 |
| [4] |
M. Giannerini, M. Fañanás-Mastral, B.L. Feringa, Nat. Chem. 5 (2013) 667. DOI:10.1038/nchem.1678 |
| [5] |
M. Giannerini, V. Hornillos, C. Vila, M. Fañanás-Mastral, B.L. Feringa, Angew. Chem. Int. Ed. 52 (2013) 13329-13333. DOI:10.1002/anie.201306427 |
| [6] |
V. Hornillos, M. Giannerini, C. Vila, M. Fañanás-Mastral, B.L. Feringa, Org. Lett. 15 (2013) 5114-5117. DOI:10.1021/ol402408v |
| [7] |
C. Vila, M. Giannerini, V. Hornillos, M. Fañanás-Mastral, B.L. Feringa, Chem. Sci. 5 (2014) 1361-1367. DOI:10.1039/c3sc53047g |
| [8] |
C. Vila, V. Hornillos, M. Giannerini, M. Fañanás-Mastral, B.L. Feringa, Chem. -Eur. J. 20 (2014) 13078-13083. DOI:10.1002/chem.201404398 |
| [9] |
L.M. Castelló, V. Hornillos, C. Vila, et al., Org. Lett. 17 (2015) 62-65. DOI:10.1021/ol5032409 |
| [10] |
D. Heijnen, V. Hornillos, B.P. Corbet, M. Giannerini, B.L. Feringa, Org. Lett. 17 (2015) 2262-2265. DOI:10.1021/acs.orglett.5b00905 |
| [11] |
E.B. Pinxterhuis, M. Giannerini, V. Hornillos, B.L. Feringa, Nat. Commun. 7 (2016) 11698. DOI:10.1038/ncomms11698 |
| [12] |
D. Heijnen, F. Tosi, C. Vila, et al., Angew. Chem. Int. Ed. 56 (2017) 3354-3359. DOI:10.1002/anie.201700417 |
| [13] |
C. Bolm, J. Legros, J. Le Paih, L. Zani, Chem. Rev. 104 (2004) 6217-6254. DOI:10.1021/cr040664h |
| [14] |
Z. Jia, Q. Liu, X.S. Peng, H.N.C. Wong, Nat. Commun. 7 (2016) 10614. DOI:10.1038/ncomms10614 |
| [15] |
X.L. Lu, M. Shannon, X.S. Peng, H.N.C. Wong, Org. Lett. 21 (2019) 2546-2549. DOI:10.1021/acs.orglett.9b00394 |
| [16] |
Q. Liu, Z.Y. Wang, X.S. Peng, H.N.C. Wong, J. Org. Chem. 83 (2018) 6325-6333. DOI:10.1021/acs.joc.8b00510 |
| [17] |
Z. Zhong, Z.Y. Wang, S.F. Ni, et al., Org. Lett. 21 (2019) 700-704. DOI:10.1021/acs.orglett.8b03893 |
| [18] |
W.F. Bailey, T.V. Ovaska, J. Am. Chem. Soc. 115 (1993) 3080-3090. DOI:10.1021/ja00061a006 |
| [19] |
J. Zhao, Z. Xu, K. Oniwa, et al., Angew. Chem. Int. Ed. 55 (2016) 259-263. DOI:10.1002/anie.201507794 |
 2019, Vol. 30
2019, Vol. 30 

