b College of Chemistry Biology and Environment, Yuxi Normal University, Yuxi 653100, China
Biomimetic nanochannels have gained remarkable attention because of their great stability, easy preparation and functionalization [1]. The biomimetic nanochannels provided convenient artificial platforms to study and simulate biological processes of living organisms [2]. By using chemical receptors, nanochannels have been engineered to respond to many stimuli [3, 4], such as ions [5-13], thermal [14-16], light [17-19], pressure [20], pH [21-23], molecules [24-28]. Moreover, since life functions rely on synergistic effect of several molecules [29-31], it is necessary to develop multiple responsive biomimetic nanochannels with differential response to multiple molecules. In the area of chemosensors, several types of multi-functional sensing modes have been developed, such as dual-responsive receptors [32-34], ion-pair sensors [35, 36], relay recognition systems [37-39] and cascade recognition [40-43]. However, it is still a great demand to exploit multiple responsive biomimetic nanochannels, although there are some reports on sequential recognition nanochannels [44, 45] and nanofluidic IMP (implication) logic device [46].
For example, boron is an essential nutrient for most organisms [47]. Boron is not only important to animals [48, 49], but also essential for normal growth of higher plants [50, 51]. The affinity between borate and cis-hydroxyl compounds may account for the mechanism by which boron implements some of its biological functions [52, 53]. Therefore, it is fascinating to construct nanodevices that can respond to both borate and cis-hydroxyl compounds.
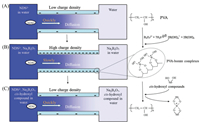
Here, we developed biomimetic nanochannels based on solid nanochannels for cascade response of borate and cis-hydroxy compounds, by using layer-by-layer self-assembly of poly(sodium 4-styrenesulfonate) (PSS), poly(diallyl-dimethyl ammonium chloride) (PDADMAC) and polyvinyl alcohol (PVA) in the nanochannels of track-etched polycarbonate (TEPC) membranes. PVA has binding sites for borate to form negatively charged PVA-borate complexes (Fig. 1) [54]. If there are cis-hydroxyl compounds, the PVA-borate complexes will be destroyed and then borate will leave the surface of nanochannels along with cis-hydroxyl compounds, due to the stronger binding affinity between borate and cis-hydroxyl compounds (Figs. 1B and C). During the cascade response of borate and cis-hydroxyl compounds, negative charge density on the modified nanochannels will be changed from low to high and then back to low. To monitor the recognition processes, the transport of 1, 5-naphthalene disulfonate (NDS2-) was utilized [55, 56].
|
Download:
|
| Fig. 1. Schematic of NDS2- transport across the charged nanochannel membranes. (A) The transport of NDS2- in water; (B) The transport of NDS2- after the addition of borate; (C) The transport of NDS2- after addition of cis-hydroxyl compounds in the presence of borate. | |
Although the PVA modified nanochannels show low negative charge density since a small amount of borate bind to PVA during the assemble process (Fig. S1 in Supporting information), NDS2- can diffuse through the nanochannels rapidly (Fig. 1A). Then, the formation of negatively charged PVA-borate complexes increases the negative charge density of the nanochannels (Fig. 1B). The transport of NDS2- is obstructed due to electrostatic repulsion, which implies that the nanochannels are responsive to borate. Subsequently, the addition of cis-hydroxyl compounds will carry off borate from the inner wall of nanochannels due to the stronger binding affinities between borate and cis-hydroxyl compounds, resulting in the decrease of negative charge density on nanochannels and then enhancing the transport of NDS2- (Fig. 1C). At this point, the nanochannels have performed cascade response to borate and cis-hydroxyl compounds, and the nanochannels return to initial state and ready for the next cycle of cascade response.
We began the study with the modification of 30 nm TEPC membranes (Fig. S2 in Supporting information). The modified membranes showed smaller pore diameters (Fig. S3 in Supporting information). The TEM images of the liberated multilayer nanostructures clearly showed that the modified multilayers were assembled in the interior of the nanochannels (Fig. S4 in Supporting information). To confirm whether the charge density could be affected by Na2B4O7 and cis-hydroxyl compounds, the zeta potentials of PVA solution, PVA/Na2B4O7 mixed solution, PVA/ Na2B4O7/mannitol mixed solution were characterized. As shown in Fig. S5 (Supporting information), PVA exhibited negative zeta potential; after incubating with Na2B4O7, the negative zeta potential was enhanced; and the negative zeta potential was decreased with the addition of mannitol.
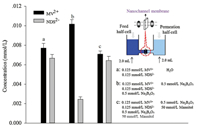
In addition, the transport of positively and negatively charged dyes (MV2+, NDS2-) in nanochannels was investigated under different conditions (Fig. 2). The experimental device was the same as reported previously [55, 56]. In brief, it is a U-tube setup and the two half-cells were separated by nanochannel membranes. The transport speed of MV2+ was slightly faster than that of NDS2- in water (condition a), implying that the nanochannels showed low negative charge density. In 0.5 mmol/L Na2B4O7 solution (condition b), the transport of MV2+ was significantly enhanced, while the transport of NDS2- was obviously obstructed. This was because of a large amount of negatively charged borate bound to the PVA on the surface of nanochannels. Under condition c, i.e. 0.5 mmol/L Na2B4O7 coexisted with 50 mmol/L mannitol, the transport behaviors of the two dyes were almost the same as that under condition a, i.e. the nanochannels return to initial state due to the leaving of borate induced by higher affinity between borate and mannitol. These results were consistent with Fig. 1. Since the concentration changes of NDS2- in permeation half-cell was higher than that of MV2+, NDS2- was used in the following investigations.
|
Download:
|
| Fig. 2. Transports of MV2+ and NDS2- under different conditions. The vertical axis represents concentration of MV2+ (black) or NDS2- (grey) in the permeation halfcell. The error bars indicate the standard deviations of three experiments. MV2+: methylviologen. | |
The nanochannel membranes showed good selectivity to borate, only the addition of borate obstructed the transport of NDS2- significantly (Fig. S6 in Supporting information). Although the solution of Na2B4O7 was alkaline (pH 9.0), the nanochannel membranes did not response to pH 9 NaOH solution (Fig. S7 in Supporting information). The response of modified nanochannels to different concentration of borate was investigated (Fig. S8A in Supporting information). When the concentration of borate increased, the transport of NDS2- was obstructed and reached the least value in 0.1 mmol/L Na2B4O7. However, with the further increase of the concentration of borate, the transport of NDS2- was increased again because the high ionic strength induced the shrink of confined-diffusion region and the expansion of the freediffusion region [57]. In addition, the transport of NDS2- was increased over time (Fig. S9 in Supporting information). Furthermore, the reversibility of PVA modified nanochannels was studied by altering the solution in U-tube setup (Fig. S8B in Supporting information). The concentration of NDS2- in permeation cell increased and decreased in the presence and absence of borate, respectively.
According to the model in Fig. 1, the borate-PVA modified nanochannels can response to cis-hydroxyl compounds. Therefore, the transport of NDS2- across nanochannels was respectively investigated using four cis-hydroxyl compounds. In the presence of 0.1 mmol/L Na2B4O7, the addition of cis-hydroxyl compounds enhanced the transport of NDS2- especially for mannitol and sorbitol (Fig. S10A in Supporting information), which can be explained as follows: the more hydroxyl groups, the greater the possibility of o-hydroxyl in the cis-conformation, so mannitol and sorbitol showed higher affinity to the borate. The concentration of NDS2- in permeate half-cell was increased over time (Fig. S11 in Supporting information). As the control, the case without borate was also investigated, the transport of NDS2- was quick and there was no distinction among the cis-hydroxyl compounds (Fig. S10B in Supporting information).
The response of borate-PVA modified nanochannels to different concentrations of mannitol was investigated (Fig. S12A in Supporting information). With the increase of mannitol concentration, the transport of NDS2- enhanced gradually, which was consistent with the hypotheses in Fig. 1. When the concentration of mannitol was 50 mmol/L, the transport of NDS2- was approximately equal to the situation of water in Fig. S10B. This result implied that the borates on the inner surface of nanochannels were almost carried off by mannitol, and the nanochannels were almost neutral. Meanwhile, the borate-PVA modified nanochannels also displayed good reversible response to mannitol (Fig. S12B in Supporting information).
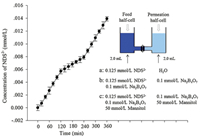
To directly demonstrate the cascade response of PVA modified nanochannels to borate and mannitol, we monitored the transport of NDS2- during the whole response process. As shown in Fig. 3, period a (0–120 min) represented NDS2- transport under water; period b (120–240 min) represented NDS2- transport after adding borate; period c (240–360 min) represented NDS2- transport after the addition of 50 mmol/L mannitol. Comparing period a, b and c, the slope of the curve firstly decreased and then increased, i.e. the transport of NDS2- was firstly obstructed and then enhanced, indicating the cascade response of PVA modified nanochannels to borate and mannitol.
|
Download:
|
| Fig. 3. Time-response of PVA modified nanochannel membranes to borate and mannitol. The vertical axis represents concentration of NDS2- in the permeation half-cell. The error bars indicate the standard deviations of three experiments. | |
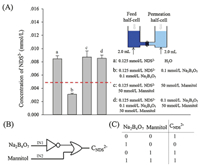
The PVA modified nanochannels with reversible cascade response can also be regarded as logic devices with IMP property (Fig. 4). The borate and mannitol were defined as the input signal "IN1" and "IN2", respectively; and the concentration of NDS2- in the permeation half-cell was defined as the output signal. The presence of borate or mannitol was defined as "1" for inputs; otherwise, the inputs defined as "0". For output, if the concentration of NDS2- in the permeation half-cell was higher than 0.005 mmol/L, then the output was defined as "on" and "1"; otherwise, the output was defined as "off" and "0". From the logic gate functions, the output "0" was observed only in the presence of borate (IN1 = 1) and in the absence of mannitol (IN2 = 0). In other input conditions, the system was in the "open" state. Therefore, this is an IMP logic gate.
|
Download:
|
| Fig. 4. (A) The concentration of NDS2- in the permeate cell under different conditions. The error bars indicate the standard deviations of three experiments. (B) IMP logic gate was represented using a conventional gate notation. (C) Truth table for the IMP logic gate: borate and mannitol were inputs, and the concentration of NDS2- was the output signal. | |
In summary, we have demonstrated a nanofluidic cascade response device based on the nanochannels of TEPC membranes functionalized with PVA. First, the borate bound with PVA to form negatively charged PVA-borate complexes that obstructed the transport of NDS2- in the nanochannels, and the transport of NDS2- reached the least value in the presence of 0.1 mmol/L Na2B4O7. Subsequently, the addition of cis-hydroxyl compounds induced leaving of borate due to the stronger binding affinities between borate and cis-hydroxyl compounds, which reduced the negative charge density on nanochannels and enhanced the transport of NDS2-. In addition, the response of nanochannels to borate and mannitol can be repeated four times with the same nanochannel membrane. Moreover, such cascade response model also showed the properties of IMP logic gate. Therefore, our study may be helpful to investigate and simulate the physiological functions of boron in living organisms and has the potential of designing and constructing new biosensors.
AcknowledgmentsThe authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21675047, 21735002 and 21521063), the Key Point Research and Invention Program of Hunan Province (No. 2017DK2011).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2019.04.003.
| [1] |
Z. Long, S. Zhan, P. Gao, et al., Anal. Chem. 90 (2018) 577-588. DOI:10.1021/acs.analchem.7b04737 |
| [2] |
X. Hou, W. Guo, L. Jiang, Chem. Soc. Rev. 40 (2011) 2385-2401. DOI:10.1039/c0cs00053a |
| [3] |
G. Pérez-Mitta, A. Albesa, C. Trautmann, M. Toimil-Molares, O. Azzaroni, Chem. Sci. 8 (2017) 890-913. DOI:10.1039/C6SC04255D |
| [4] |
B. Viloznya, A. Wollenberg, P. Actis, et al., Nanoscale 5 (2013) 9214-9221. DOI:10.1039/c3nr02105j |
| [5] |
P. Gao, Q. Ma, D. Ding, Nat. Commun. 9 (2018) 4557-4568. DOI:10.1038/s41467-018-06873-z |
| [6] |
X. Li, T. Zhai, P. Gao, Nat. Commun. 9 (2018) 40-50. DOI:10.1038/s41467-017-02447-7 |
| [7] |
H. Wang, S. Hou, Q. Wang, et al., J. Mater. Chem. B 3 (2015) 1699-1705. DOI:10.1039/C4TB01804D |
| [8] |
M. Ali, I. Ahmed, P. Ramirez, et al., Nanoscale 8 (2016) 8583-8590. DOI:10.1039/C6NR00292G |
| [9] |
H. Chinen, K. Mawatari, Y. Pihosh, et al., Angew. Chem. Int. Ed. 51 (2012) 3573-3577. DOI:10.1002/anie.201200576 |
| [10] |
G. Xie, K. Xiao, Z. Zhang, et al., Angew. Chem. Int. Ed. 54 (2015) 13664-13668. DOI:10.1002/anie.201505269 |
| [11] |
G. Pérez-Mitta, A. Albesa, W. Knoll, et al., Nanoscale 7 (2015) 15594-15598. DOI:10.1039/C5NR04645A |
| [12] |
Q. Liu, K. Xiao, L. Wen, et al., J. Am. Chem. Soc. 137 (2015) 11976-11983. DOI:10.1021/jacs.5b04911 |
| [13] |
Q. Zhai, S. Zhang, H. Jiang, et al., J. Mater. Chem. B 2 (2014) 6371-6377. DOI:10.1039/C4TB00844H |
| [14] |
W. Guo, H. Xia, F. Xia, et al., ChemPhysChem 11 (2010) 859-864. DOI:10.1002/cphc.200900989 |
| [15] |
B. Yameen, M. Ali, R. Neumann, et al., Small 5 (2009) 1287-1291. DOI:10.1002/smll.200801318 |
| [16] |
I. Lokuge, X. Wang, P. Bohn, Langmuir 23 (2007) 305-311. DOI:10.1021/la060813m |
| [17] |
M. Zhang, Z. Meng, J. Zhai, L. Jiang, Chem. Commun. 49 (2013) 2284-2286. DOI:10.1039/c2cc38405a |
| [18] |
M. Zhang, X. Hou, J. Wang, et al., Adv. Mater. 24 (2012) 2424-2428. DOI:10.1002/adma.201104536 |
| [19] |
L. Wen, Q. Liu, J. Ma, et al., Adv. Mater. 24 (2012) 6193-6198. |
| [20] |
W. Lan, D. Holden, H. White, J. Am. Chem. Soc. 133 (2011) 13300-13303. DOI:10.1021/ja205773a |
| [21] |
G. Perez-Mitta, W. Marmisollé, C. Trautmann, M. Toimil-Molares, O. Azzaroni, J. Am. Chem. Soc. 137 (2015) 15382-15385. DOI:10.1021/jacs.5b10692 |
| [22] |
G. Pérez-Mitta, L. Burr, J. Tuninetti, et al., Nanoscale 8 (2015) 1470-1478. |
| [23] |
F. Gilles, M. Tagliazucchi, O. Azzaroni, I. Szleifer, J. Phys. Chem. C 120 (2016) 4789-4798. |
| [24] |
R. Wei, V. Gatterdam, R. Wieneke, R. Tampé, U. Rant, Nature Nanotech. 102 (2012) 257-263. |
| [25] |
Z. Sun, F. Zhang, X. Zhang, et al., Chem. Commun. 51 (2015) 4823-4826. DOI:10.1039/C4CC09012H |
| [26] |
H. Okuyama, Y. Oshiba, H. Ohashi, T. Yamaguchi, Small 14 (2018) 1702267-1702272. DOI:10.1002/smll.201702267 |
| [27] |
Q. Nguyen, M. Ali, R. Neumann, W. Ensinger, Sens. Actuators B-Chem. 162 (2012) 216-222. DOI:10.1016/j.snb.2011.12.070 |
| [28] |
S. Zhang, A. Bao, T. Sun, E. Wang, J. Wang, Biosens. Bioelectron. 63 (2015) 287-293. DOI:10.1016/j.bios.2014.07.062 |
| [29] |
T. Tanoue, E. Nishida, Cell Signal. 15 (2003) 455-462. DOI:10.1016/S0898-6568(02)00112-2 |
| [30] |
T. Pawson, Nature 373 (1995) 573-580. DOI:10.1038/373573a0 |
| [31] |
K. Jin, Z. Luo, B. Zhang, Z. Pang, Acta Pharm. Sin. B 8 (2018) 23-33. DOI:10.1016/j.apsb.2017.12.002 |
| [32] |
Y. Cheng, C. Sun, R. Liu, et al., Angew. Chem. Int. Ed. 58 (2019) 5049-5053. DOI:10.1002/anie.201901527 |
| [33] |
L. Qu, C. Yin, F. Huo, et al., Sens. Actuators B-Chem. 191 (2014) 158-164. DOI:10.1016/j.snb.2013.09.114 |
| [34] |
X. He, V. Yam, Org. Lett. 13 (2011) 2172-2175. DOI:10.1021/ol200277n |
| [35] |
S. Kim, J. Sessler, Chem. Soc. Rev. 39 (2010) 3784-3789. DOI:10.1039/c002694h |
| [36] |
F. Otón, M. González, A. Espinosa, et al., J. Org. Chem. 77 (2012) 10083-10092. DOI:10.1021/jo301570u |
| [37] |
Y. Cheng, J. Dai, C. Sun, et al., Int. Ed. 57 (2018) 3123-3127. DOI:10.1002/anie.201712803 |
| [38] |
M. Dong, Y. Peng, Y. Dong, N. Tang, Y. Wang, Org. Lett. 14 (2012) 130-133. DOI:10.1021/ol202926e |
| [39] |
Y. Peng, Y. Dong, M. Dong, Y. Wang, J. Org. Chem. 77 (2012) 9072-9080. DOI:10.1021/jo301548v |
| [40] |
X. Wang, J. Dai, X. Min, Anal. Chem. 90 (2018) 8162-8169. DOI:10.1021/acs.analchem.8b01456 |
| [41] |
L. Wang, Y. Tian, L. Ding, et al., RSC Adv. 7 (2017) 16916-16923. DOI:10.1039/C7RA00846E |
| [42] |
G. Wang, H. Chen, Y. Chen, N. Fu, Sens. Actuators B-Chem. 233 (2016) 550-558. DOI:10.1016/j.snb.2016.04.119 |
| [43] |
J. Wu, Y. Gao, J. Lu, J. Hu, Y. Ju, Sens. Actuators B-Chem. 206 (2015) 516-523. DOI:10.1016/j.snb.2014.09.097 |
| [44] |
Y. Zhang, R. Zhou, Z. Zhao, et al., ChemPhysChem 18 (2017) 253-259. |
| [45] |
C. Han, H. Su, Z. Sun, et al., Chem.-Eur. J. 19 (2013) 9388-9395. DOI:10.1002/chem.201300200 |
| [46] |
Y. Jiang, N. Liu, W. Guo, F. Xia, L. Jiang, J. Am. Chem. Soc. 134 (2012) 15395-15401. DOI:10.1021/ja3053333 |
| [47] |
W. Frommer, V. Nicolaus, Nature 420 (2002) 282-283. DOI:10.1038/420282a |
| [48] |
M. Kabu, T. Civelek, Revue Méd. Vét. 163 (2012) 419-430. |
| [49] |
C. Hunt, J. Trace Elem. Med. Biol. 26 (2012) 157-160. DOI:10.1016/j.jtemb.2012.03.014 |
| [50] |
M. Tanaka, T. Fujiwara, Pflugers Arch-Eur. J. Physiol. 456 (2008) 671-677. DOI:10.1007/s00424-007-0370-8 |
| [51] |
K. Haseeb, J. Zhong, K. Peng, Biol. Trace Elem. Res. 2 (2018) 1-21. |
| [52] |
M. Kabu, M. Akosman, Rev. Environ. Contam. Toxicol. 225 (2013) 57-75. |
| [53] |
L. Bolaños, K. Lukaszewski, I. Bonilla, D. Blevins, Plant Physiol. Biochem. 42 (2004) 907-912. DOI:10.1016/j.plaphy.2004.11.002 |
| [54] |
U. Manna, S. Patil, ACS Appl. Mater. Interfaces 2 (2010) 1521-1527. DOI:10.1021/am100139j |
| [55] |
M. Yang, X. Yang, K. Wang, et al., Anal. Chem. 87 (2015) 1544-1551. DOI:10.1021/ac503813r |
| [56] |
M. Yang, X. Yang, Q. Wang, et al., RSC Adv. 4 (2014) 26729-26737. DOI:10.1039/C4RA02856B |
| [57] |
W. Chen, Z. Wu, X. Xia, J. Xu, H. Chen, Angew. Chem. Int. Ed. 49 (2010) 7943-7947. DOI:10.1002/anie.201002711 |
 2019, Vol. 30
2019, Vol. 30 

