In recent years, photoredox catalysis has received great attention as a powerful strategy for the activation of organic molecules [1]. Metal complexes, organic dyes and other small molecules have been used to convert visible light into chemical energy by engaging in single-electron transfer with discrete organic substrates [2-4]. In this category, [Ru(bpy)3]2+ and its derivatives have been demonstrated as a family of most robust catalysts to mediate the transfer of electrons between different substrates and intermediates [5]. Covalent attachment of the [Ru (bpy)3]2+ moiety to solid porous materials such as regular metalorganic framework or supramolecular organic framework (SOF) has been revealed to achieve both high catalysis efficiency and recyclable use of the catalysts [6, 7]. We have previously developed a general self-assembly strategy for the generation of SOFs from multiarmed building blocks and cucurbit[8]uril (CB[8]) in water [8]. By using a hexaarmed [Ru(bpy)3]2+-derived precursor, we have assembled the first [Ru(bpy)3]2+-cored supramolecular metal organic framework (SMOF-1) [9], which adsorbs anionic Wells-Dawson-type polyoxometalate clusters to enable visible light photocatalysis of the reduction of proton to hydrogen gas.
Reduction of organic azides is one of the most important protocols for the preparation of amines owing to the easy accessibility of azides from respective halides, alcohols, and sulfonates [10]. Several groups have investigated homogeneous visible light photocatalysis of the reduction of phenyl azides to the corresponding amines [11]. We recently reported that [Ru(bpy)3]2+-attached tetraphenylmethane-derived SOF (SOF-CH = N-[Ru(bpy)3]) could function as heterogeneous recyclable catalyst for this conversion [9]. We herein describe the visible light-induced recyclable heterogeneous photocatalysis of SMOF-1 for the reduction of phenyl, benzyl and 2-phenylethyl azides to the corresponding amines. We further demonstrate that benzyl and 2-phenylethyl azides that bear a methyl ester group at the ortho-position of the benzene ring undergo can cascade reactions to afford the corresponding lactams in good to excellent yields.
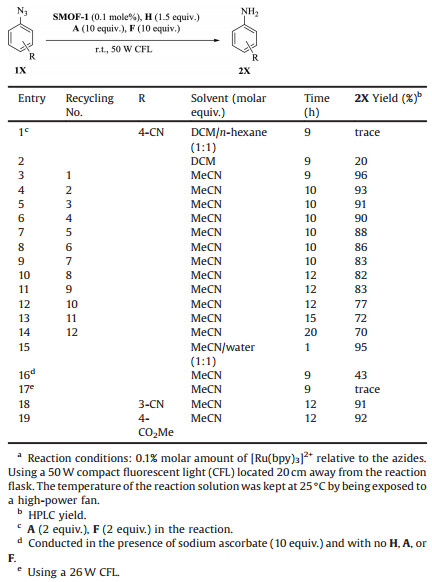
SMOF-1 contains a [Ru(bpy)3]2+ core whose 2, 2'-bipyriding ligands are covalently modified to form peripheral arms for the formation of the regular porous framework [9]. Because the dynamic hydrazine bond of SOF-CH = N-[Ru(bpy)3] underwent partial decomposition during the recycling photocatalysis [12], we investigated the catalysis efficiency of SMOF-1 for the reduction of phenyl azides. Previous study with SOF-CH = N-[Ru(bpy)3] showed that phenyl azides that bear electron-withdrawing groups on the benzene ring gave rise to higher yield of amines [9]. Thus, four substrates that bear electron-withdrawing groups were used for the present study. As for earlier reports, the reactions were conducted in the presence of Hantzsch ester (H), i-Pr2NEt (A) and HCO2H (F) and the flask was irradiated with a 50 W compact fluorescent light (CFL). The reaction conditions were first screened at room temperature using 4-cyanophenyl azide 1X (R=4-CN) as model substrate. In solvents of low polarity such as n-hexane or dichloromethane (DCM), no or low yield of 4-cyano-substituted amine was formed (entries 1 and 2, Table 1). In acetonitrile, the reaction occurred smoothly. After 9 h, thin layer chromatography (TLC) showed that the azide had consumed completely (entry 3, Table 1), and the yield of the amine was determined to be 96% by HPLC. The reaction conditions, that is, SMOF-1(0.1%), H (1.5 equiv.), A (10 equiv.) and F (10 equiv.) in acetonitrile, were thus chosen for other substrates. In the mixture of acetonitrile and water (1:1, v/v), the reaction took place much more quickly, and after 1 h the azide was consumed to give the amine in 95% yield (entry 15, Table 1). This result might be attributed to the partial dissolution of SMOF-1 because the solution was orange, which should correspond to the absorption of the [Ru(bpy)3]2+ complex. With sodium ascorbate as reducing agent [13], the amine was obtained in 43% yield (entry 16, Table 1). When the reaction was applied to a 26 W CFL, only a trace of the amine was formed (entry 17, Table 1). Other azides such as those with 3-CN or 4-CO2Me being on the benzene ring could also be reduced under similar conditions to afford the corresponding amine derivatives in excellent yields (entries 18 and 19, Table 1).
|
|
Table 1 Visible light-induced reduction of phenyl azides 1X to amines 2X using SMOF-1 as photocatalyst.a |
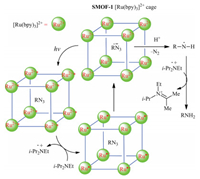
The reaction was also investigated with control photocatalyst [Ru(bpy)3]Cl2. Compared with the above results (entries 2-4, Table 1), this homogeneous complex catalyst (0.1 mol% relative to the azide) exhibited notably decreased activity. After 9 h, the yield of the amine was 17% and 56%, respectively, when the reaction was conducted in dichloromethane or acetonitrile, whereas in binary acetonitrile and water (1:1), the reaction afforded 70% of the amine after 1 h. Considering the heterogeneity of SMOF-1, we attributed its increasedcatalysis ability toits porous structure, through which the eight [Ru(bpy)3]2+ units that formed a cubic cage could jointly mediate the reduction of an azide and the intermediates that were located in the same cage (Fig. 1, vide infra).
|
Download:
|
| Fig. 1. Proposed synergistic effect of the [Ru(bpy)3]2+ units of SMOF-1 that form the cubic cages for the photoreduction of organic azides to amines. | |
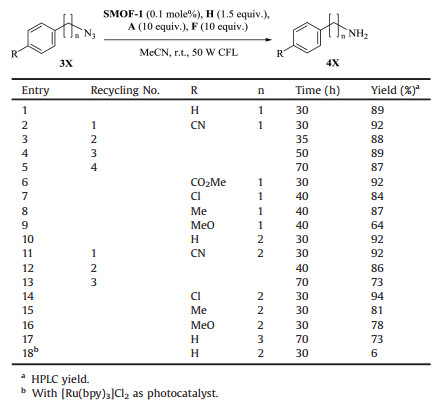
Visible light-induced photocatalysis of SMOF-1 for benzyl azides 3X (n=1) to form the corresponding amines 4X (n=1) were then conducted in acetonitrile under the conditions shown in entry 3, Table 1. The results are shown in Table 2. For all the six investigated substrates, longer time was needed for their complete consumption, which is consistent with their decreased ability of accepting electron as compared with phenyl azides. For benzyl azide and its derivatives that bear the CN, CO2Me, Cl or Me group at the para-position of the benzene ring (entries 2 and 6-9, Table 2), the reaction affordedtheaminesin highyields (84%-92%), whereas for the azide that bears OMe (entry 9, Table 2), a typical electronrich group, at the para-position of the benzene ring, the corresponding amine was obtained in relatively low yield of 64%, reflecting that strong electron-rich group is unfavourable for the reaction by reducingthe electron-acceptingability of the azide. With [Ru(bpy)3]Cl2 of the same molaramount as catalyst, after 30 h of irradiation, the reaction of (4-cyanophenyl)methyl azide (3X, R = CN, n=1) gave rise to the corresponding amine in only 10% yield, which again supported the increased activity of the [Ru (bpy)3]2+ incorporated in the framework.
|
|
Table 2 Visible light-induced reduction of aliphatic azides 3X toamines 4X using SMOF-1 as photocatalyst. |
Encouraged by the above results for benzyl azides, we also studied the photoreduction of 2-phenylethyl azides 3X (n=2) under the above reaction conditions for benzyl azides. After about 30 h, the substrates were consumed and all the reactions gave rise to high yields (78%-94%) (entries 10, 11 and 14-16, Table 2), even though the reaction yield of the substrate that has a MeO group at the para-position of the benzene ring was also the lowest. When the identical reaction conditions were applied for 3-phenylpropyl azide 3X (n=3), the corresponding amine was obtained in 73% yield after long time of irradiation (70 h). All the results showed the generality of the photocatalysis approach for the reduction of aliphatic azides to amines. For 2-phenylethyl azide, when [Ru(bpy)3]Cl2 was used, the amine was generated in the very low yield of 6%.
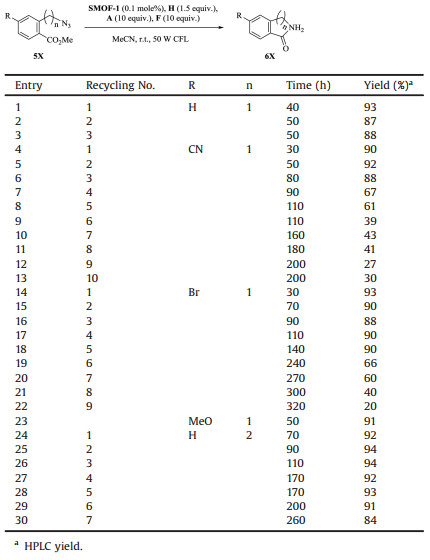
It has been reported that methyl 2-(aminomethyl) and 2-(2-aminoethyl)benzoates undergo intramolecular substitution to yield the corresponding lactams [14, 15], a family of organic compounds that may exhibit important biological activity [16]. We thus further studied the cascade reaction of azides 5X that bear a CO2Me group at the ortho-position of their benzene ring. Although the reaction times were different for the azides to be consumed, all the reactions of the five studied azides, that bears no or one electron-deficient or rich group, produced the expected lactams with very high yields (90%-93%) (entries 1, 4, 14, 23 and 24, Table 3). When control [Ru(bpy)3]Cl2 was used as catalyst, after irradiatingfor 40, 30, 30 or 70 h, the four azides (5X: n=1, R = H, CN, Br, and n=2, R = H) could afford the corresponding lactams in 3%, 2%, 2% or 8% yield, respectively.
|
|
Table 3 Visible light-induced reduction and subsequent intramolecular substitution of CO2Me-bearing aliphatic azides 5X to lactams 6X. |
The recyclability of the SMOF-1 catalyst was further studied for several azides. The catalysts were recovered by simply centrifuging and then removing the solution and used for the next step. For 1X (R=4-CN) (entries 3-14, Table 1), the catalyst was still able to exhibit considerable activity after 12 runs, even though the reaction needed longer time, which well reflected the high stability of SMOF-1 as a heterogeneous catalysis. Synchrotron X-ray powder diffraction experiment for the recovered SMOF-1 sample after 12 runs revealed a broad but discernible peak (Fig. S1 in Supporting information), which corresponded to the {100} spacing of the calculated value of the modelled cubic framework [9]. This observation supported that the sample still kept its regularity after repeated irradiation. The slow decrease of the catalysis activity with the increase of the run time may be mainly attributed to its partial loss during the recovery process, even though the decomposition of the framework or the complex molecule cannot be excluded.
The recycling use of SMOF-1 was then investigated for several other azides. Generally, repeated use of the recovered catalysis required elongated irradiation for achieving high yield of the corresponding products.For 3X(R=CN, n=1)(entries2-5, Table 2), after 4 runs, the yield of the amine was still as high as 87% by elongating the irradiation time from 30 h to 70 h. For 3X (R=CN, n=2) (entries 11-13, Table 2), the catalyst could also be repeatedly used. However, after 3 runs, the yield of the amine decreased considerably from 92% to 73%. Concerning the cascade reactions of the CO2Me-bearing azides, the reaction 5X (R = H, n=1) gave rise to the lactam in very high comparable yields for 3 runs (entries 1-3, Table 3). For 5X (R=CN, n=1), the first 3 runs also afforded the lactam product in comparable yields (entries 4-6, Table 3), but further repeated use of the recovered catalyst led to continued decrease of the reaction yield from 67% (run 4) to 30% (run 10) (entries 7-13, Table 3). For 5X (R=Br, n=1), the first 5 runs could obtain high yield of the lactam (entries 14-18, Table 3), and from the sixth tothe tenth, the yield decreased substantially from 66% to 20% (entries 19-22, Table 3). For 5X (R = H, n=2), 7 runs were conducted and the reactions all generated the lactam in high yield (entries 24-30, Table 3).
The mechanism of [Ru(bpy)3]2+-catalysed photoreduction of organic azides to amines in organic solvents in the presence of i-Pr2NEt has been investigated previously by Chen and co-workers [11a]. It is reasonable to propose that the present reactions of azides 1X, 3X and 5X proceeded throughthe same mechanism. The increased activityof the [Ru(bpy)3]2+ units of SMOF-1, as compared with [Ru(bpy)3]Cl2 control, reflects the synergistic feature of the eight [Ru(bpy)3]2+ units that form one cubic cage (Fig. 1), which facilitated electron transfer from [Ru(bpy)3]+ to azide. The result also implied that porous SMOF-1 is highly transparent and thus could allow for efficient penetration of visible light.
In conclusion, we have demonstrated that [Ru(bpy)3]2+-cored supramolecular organic framework can be used as heterogeneous catalysis for the visible light-induced photoreduction of various organic azides to the corresponding amines, which can undergo cascade reactions to afford discrete lactams through subsequent ester substitution. Generally, the framework-styled catalyst exhibited very good recyclability, which can allow for more than 10 runs of repeating use and considerable retainment of the photocatalysis activity. Moreover, compared with the homogeneous control [Ru(bpy)3]Cl2, the new framework-styled catalyst exhibits remarkably increased catalysis activity due to the synergistic effect of the [Ru(bpy)3]2+ units that form the cubic cages to host the substrates or intermediates. Thus, this new selfassembled photocatalysis system will be further explored for many other important reactions.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21432004 and 21890732). We also thank Shanghai Synchrotron Radiation Facility (beamlines BL16B1 and BL14B1) for providing the beam time for this work.
| [1] |
C.R.J. Stephenson, T.P. Yoon, D.W.C. MacMillan (Eds.), Visible Light Photocatalysis in Organic Chemistry, Wiley-VCH, Weinheim, 2018, p. 456.
|
| [2] |
(a) J.M.R. Narayanam, C.R.J. Stephenson, Chem. Soc. Rev. 40 (2011) 102-113; (b) J. Xuan, W.J. Xiao, Angew. Chem. Int. Ed. 51 (2012) 6828-6838; (c) Y. Xi, H. Yi, A. Lei, Org. Biomol. Chem. 11 (2013) 2387-2403; (d) L. Zhang, E. Meggers, Acc. Chem. Res. 50 (2017) 320-330; (e) Y. Guo, M.W. Huang, X.L. Fu, et al., Chin. Chem. Lett. 28 (2017) 719-728; (f) B. Yang, X. Ren, T. Shen, Z. Lu, Chin. J. Chem. 36 (2018) 1017-1023; (g) B. Chen, L.Z. Wu, C.H. Tung, Acc. Chem. Res. 51 (2018) 2512-2523; (h) W. Yu, Y. Ouyang, X.H. Xu, F.L. Qing, Chin. J. Chem. 36 (2018) 1024-1030; (i) D. Wang, L. Zhang, S. Luo, Chin. J. Chem. 36 (2018) 311-320; (j) W.J. Zhou, Y.H. Zhang, Y.Y. Gui, L. Sun, D.G. Yu, Synthesis 50 (2018) 3359-3378. |
| [3] |
(a) B. König, Eur. J. Org. Chem. (2017) 1979-1981; (b) J.J. Zhong, Q.Y. Meng, B. Chen, C.H. Tung, L.Z. Wu, Acta Chim. Sin. 75 (2017) 34-40; (c) J. Wang, B. Li, L.C. Liu, et al., Sci. China Chem. 61 (2018) 1594-1599; (d) T. Shao, Z. Jiang, Acta Chim. Sin. 75 (2017) 70-73. |
| [4] |
(a) J.J. Zhang, Y.B. Cheng, X.H. Duan, Chin. J. Chem. 35 (2017) 311-315; (b) P. Pei, F. Zhang, H. Yi, A. Lei, Acta Chim. Sin. 75 (2017) 15-21; (c) M. Liu, Y. Li, L. Yu, Q. Xu, X. Jiang, Sci. China Chem. 61 (2018) 294-299; (d) M. Zhang, R. Ruzi, N. Li, J. Xie, C. Zhu, Org. Chem. Front. 5 (2018) 749-752; (e) Y. Chen, L.Q. Lu, D.G. Yu, C.J. Zhu, W.J. Xiao, Sci. China Chem. 62 (2019) 24-57. |
| [5] |
(a) F. Teply, Coll. Czech. Chem. Commun. 76 (2011) 859-917; (b) S. Maity, N. Zheng, Synlett 23 (2012) 1851-1856; (c) Y. Yuan, W. Dong, X. Gao, et al., Chin. J. Chem. 36 (2018) 1035-1040; (d) B. Gou, C. Yang, L. Zhang, W. Xia, Acta Chim. Sin. 75 (2017) 66-69; (e) D. Wang, L. Zhang, S. Luo, Acta Chim. Sin. 75 (2017) 22-33; (f) R. Mao, L. Sun, Y.S. Wang, et al., Chin. Chem. Lett. 29 (2018) 61-64; (g) J. Zhang, Y. Chen, Acta Chim. Sin. 75 (2017) 41-48. |
| [6] |
(a) X. Yu, S.M. Cohen, Chem. Commun. 51 (2015) 9880-9883; (b) J.S. Qin, S. Yuan, C. Lollar, et al., Chem. Commun. 54 (2018) 4231-4249. |
| [7] |
Y.P. Wu, B. Yang, J. Tian, et al., Chem. Commun. 53 (2017) 13367-13370. DOI:10.1039/C7CC08824H |
| [8] |
(a) H. Wang, D.W. Zhang, X. Zhao, Z.T. Li, Acta Chim. Sin. 73 (2015) 471-479; (b) J. Tian, T.Y. Zhou, S.C. Zhang, et al., Nat. Commun. 5 (2014) 5574; (c) J. Tian, L. Chen, D.W. Zhang, Y. Liu, Z.T. Li, Chem. Commun. 52 (2016) 6351-6362; (d) H. Wang, D.W. Zhang, Z.T. Li, Acta Polym. Sin. (2017) 19-26; (e) J. Tian, H. Wang, D.W. Zhang, Y. Liu, Z.T. Li, Sci. Rev. 4 (2017) 426-436; (f) J. Tian, C. Yao, W.L. Yang, et al., Chin. Chem. Lett. 28 (2017) 798-806; (g) C. Yao, J. Tian, H. Wang, et al., Chin. Chem. Lett. 28 (2017) 893-899; (h) Y. Chen, F. Huang, Z.T. Li, Y. Liu, Sci. China Chem. 61 (2018) 979-992; (i) X.F. Li, S.B. Yu, B. Yang, et al., Sci. China Chem. 61 (2018) 830-835; (j) S.B. Yu, Q. Qi, B. Yang, et al., Small 14 (2018) 1801037. |
| [9] |
J. Tian, Z.Y. Xu, D.W. Zhang, et al., Nat. Commun. 7 (2016) 11580. DOI:10.1038/ncomms11580 |
| [10] |
S.A. Lawrence, Amines: Synthesis, Properties and Applications, Cambridge University, Cambridge, 2005, p. 382.
|
| [11] |
(a) Y. Chen, A.S. Kamlet, J.B. Steinman, D.R. Liu, Nat. Chem. 3 (2011) 146-153; (b) M. Rothlingshofer, K. Gorska, N. Winssinger, Org. Lett. 14 (2012) 482-485; (c) X.D. Xia, J. Xuan, Q. Wang, et al., Adv. Synth. Catal. 356 (2014) 2807-2812. |
| [12] |
T. Maji, A. Karmakar, O. Reiser, J. Org. Chem. 76 (2011) 736-739. DOI:10.1021/jo102239x |
| [13] |
J.B. Borak, D.E. Falvey, J. Org. Chem. 74 (2009) 3894-3899. DOI:10.1021/jo900182x |
| [14] |
J.J. Powers, D.A. Favor, T. Rankin, et al., Tetrahedron Lett. 50 (2009) 1267-1269. DOI:10.1016/j.tetlet.2008.12.099 |
| [15] |
J. Wrobel, A. Dietrich, B.J. Gorham, K. Sestanj, J. Org. Chem. 55 (1990) 2694-2702. DOI:10.1021/jo00296a028 |
| [16] |
T. Janecki, Natural Lactones and Lactams: Synthesis, Occurrence and Biological Activity, Wiley-VCH, Weinheim, 2018, p. 456.
|
 2019, Vol. 30
2019, Vol. 30