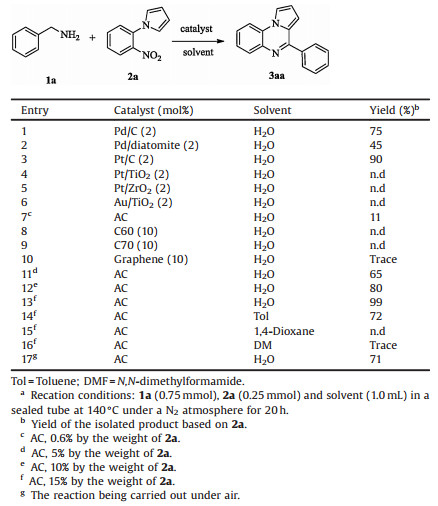
Pyrrolo[1, 2-a]quinoxalines as one of the most important nitrogen-containing heterocycles display special physiological properties and biological activities [1]. Accordingly, many methods for syntheses of pyrrolo[1, 2-a]quinoxalines and the derivatives have been developed over the past decades. The traditional approaches generally can be classified into two categories. One is the copper-catalyzed Ullmann-type coupling reaction of pyrrole analogues via an intramolecular or intermolecular cyclization [2a-c]. The other is utilizing the amines as the starting materials with aldehydes, alkyl- or aryl-acid chlorides to obtain the corresponding structural motifs [2d-f]. In 2012, a new method to construct these N-heterocycles involved an aryl nitro reduction and an aerobic oxidation of the alcohols was developed [3]. The inactive substrates benzyl alcohols were employed in this transformation. Very recently, a new strategy to synthesize indolo- and pyrrolo[1, 2-a]quinoxalines from benzylamines promoted by molecular iodine was developed by Jayaprakash et al. [4]. Despite these tremendous developments, a green and practical methodology is in highly urgent demand due to the harsh conditions involved in the previous work, including toxic solvents, large excess of metals and high reaction temperature.
As continued interest in the development of simple and general synthetic methods to access this structural moiety, on the other hand, heterogeneous catalysts have been witnessed explosive developments due to their excellent catalytic efficiency and easy recycling advantage [5]. In the past decade, chemists prefer to choose various kinds of modified carbon materials or metal supported on different templates (including activated carbon) as catalysts to construct C-N or C-C bonds directly, which was recognized as the fundamental in the synthesis of numerous natural products and pharmaceutical intermediates [6]. With great efforts focused on the heterogeneous catalysis [7], people usually use activated carbon (AC) as a heterogeneous catalyst template for its cheap and the stability in both acidic and basic media [8]. However, activated carbon as a catalyst has long been neglected in the organic reactions [9]. Additionally, water, as a cost-effective and environmentally benign solvent in synthetic chemistry, has been widely studied in our group [10] and the other groups [11]. Herein, we wish to unveil a novel method to synthesize pyrrolo [1, 2-a]quinoxalines from amines and 1-(2-nitrophenyl)pyrrole derivatives. The reaction can be catalyzed by activated carbon in water without any other additive. Mechanistically, two hydrogentransfer processes are proposed as the key steps in the catalytic cycle.
At the outset of this investigation, we selected the reaction of benzylamine (1a) with 1-(2-nitrophenyl)pyrrole (2a) as the model reaction (Table 1). In our initial studies, the reaction was carried out in water and several nano-catalysts, such as Pd/C, Pd/diatomite, Pt/C, Pt/TiO2, Pt/ZrO2 and Au/TiO2, were examined in the reaction (entries 1-6). The details were deposited in Supporting information. Interestingly, the cyclization product 3aa was produced in good yield only in the presence of activated carbon (AC). Subsequently, different carbon materials were screened and only AC could obtain 3aa in 11% yield when the dosage of AC was 0.6% of 2a by the weight (entry 7). Gratifyingly, 3aa was obtained in 99% yield when the amount of catalyst was increased to 15% of 2a by the weight (entries 11-13). Further screening the solvent showed that the yield largely decreased when several types of organic solvents were employed instead of water, affording few or a trace amount of the desired product, except for toluene with 72% yield (entries 14-16). These results indicated that the combination of activated carbon and water established a highly efficient system for 3aa synthesis. It is worth mentioning that the reaction could also perform well under air atmosphere to furnish the final product with 71% yield (entry 17).
|
|
Table 1 the one-pot synthesis of 3aa.a |
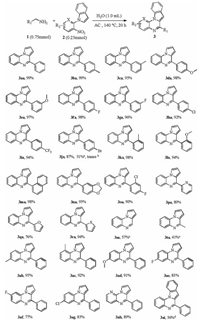
With the most efficient catalyst in hand, a broad range of amines 1a-u were prepared and employed in the reaction under the optimized conditions to explore the scope of this transformation (Scheme 1). The electronic effect of substituents on the aryl ring of the benzylamines was first examined. The results showed that the electronic properties of benzylamines have little influence on this reaction. A series of amines, bearing either electrondonating groups (p-Me and p-MeO) or electron-withdrawing group (p-F, p-Cl, p-Br, p-CF3) on the aryl ring, could be converted to the desired products (3ba-3ia) in excellent yields. The yield of 3ja could be at a good level under the catalysis of AC. In contrast, the low yield of 51% for 3ja was observed when the AC was replaced by Pt/C. Even the reaction hardly occurred under catalysis of Pd/C instead of AC. This meant that AC could efficiently restrain 4-bromobenzylamine from being reduced to benzylamine by its liberating hydrogen. As expected, the benzylamines with ortho-substituent ring could be efficiently converted into the corresponding products (3ka-3la) with high yields. 1-Naphthalenemethanamine, piperonyl-amine and 2-chloro-4-fluorobenzylamine could also perform well with excellent yields (3ma-3oa). Gratifyingly, the aromatic heterocycles such as pyridine, furan and thiophene, were well tolerated in the reaction, affording the corresponding products with good yields (3pa-3ra). To our delight, aliphatic amines could also be employed as the substrates to give the corresponding products with moderate yields (3sa-3ta).
|
Download:
|
| Scheme 1. Reaction of 1-(2-nitrophenyl)pyrrole derivatives with various amines, the isolated products' yields are based on 2. a 2 mol % Pt/C, b 2 mol% Pd/C was used instead of AC. c 2.0 mmol amine was used. d 1.0 mmol benzlyamine was used. | |
Next, we turned our attention to assess the scope of the nitro compounds. The 1-(2-nitrophenyl)pyrroles with various functional groups such as -Me, -MeO and halogen (F and Cl), even with different substitution patterns, could still react smoothly with benzylamine to furnish the desired products in good yields (3ab-3ag). To further investigate the scope of this reaction, 3-nitro-2-pyrrolopyridine was employed, obtaining 3ah with a good yield of 89%. The yield of the product 6-phenylindolo[1, 2-a]quinoxaline (3ai) was decreased to 56% perhaps due to the low nucleophilicity of the indole substituted nitrobenzene [4].
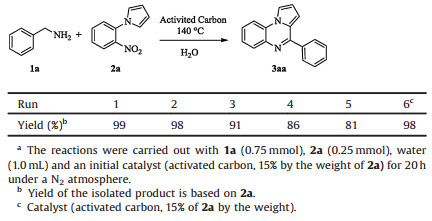
To probe the reusability of the catalyst AC, the model reaction was performed in the presence of activated carbon. The recycle of the catalyst could be realized by a simple manipulation of filtration. A little loss of the catalytic activity was observed after the fifth round, as shown in Table 2. When the additional catalyst was added to maintain the amount of AC (run 6), an excellent yield of 98% was obtained again. These results showed that the catalyst could be reused at least 6 times without the erosion of its activity.
|
|
Table 2 Recycling of activated carbon for the synthesis of pyrrolo[1, 2-a]quinoxaline.a |
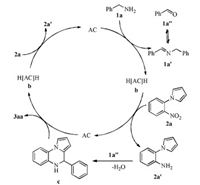
To figure out the reaction mechanism, some control experiments were conducted (Scheme 2). When the reaction was carried out in the absence of activated carbon, the product 3aa cannot be obtained (Scheme 2a), indicating that activated carbon was necessary for this reaction. Furthermore, when compound 1a" and 2a' were employed as the substrates instead of 1a and 2a under the activate carbon/water catalytic system, 89% of 3aa was obtained (Scheme 2b), but no product was observed in the absence of activated carbon (Scheme 2c). In Scheme 2d, compound 2a was employed in the reaction under H2 atmosphere, 2a' can not be generated. And when 1a was used separately under the standard condition, compound 1a' was detected with 41% yield by GC-MS. Finally, we replaced 2a with 2a' to react with 1a under the optimized condition, affording the product 3aa with 72% yield, which revealed that 2a' might be a key intermediate for this transformation.

|
Download:
|
| Scheme 2. Control experiments. Standard condition: 1a or 1a" (0.75 mmol), 2a or 2a' (0.25 mmol), activatedcarbon (15% bythe weightof 2a or 2a') and HO (1 mL) in a sealed tube at 140 ℃ under a N atmosphere for 20 h. | |
According to the control experiment results and previous reports [9a, b, c], we proposed a plausible mechanism (Scheme 3). At the very beginning of hydrogen-transfer process, the dehydrogenative oxidation of the benzylamine (1a) produces its corresponding carbonyl compound (1a") and 1-(2-nitrophenyl) pyrrole (2a) is reduced to 1-(2-aminophenyl)-1H-pyrrole (2a') by in situ generated b. Then 2a' can react rapidly with 1a" to afford the intermediate 4-phenyl-4, 5-dihydropyrrolo[1, 2-a]quinoxaline (c) under the catalysis of AC. Subsequently, c can be readily oxidized to the product 3aa in the presence of AC. Afterwards, the second hydrogen-transfer process takes place with the reduction of 2a to 2a'. In the whole catalytic cycle, the amine and the intermediate 4-phenyl-4, 5-dihydropyrrolo[1, 2-a]quinoxaline are used as reductants (hydrogen donor) once respectively and 1-(2-nitrophenyl) pyrrole is used as the oxidant (hydrogen acceptor) twice.
|
Download:
|
| Scheme 3. Proposed mechanism for the reaction. | |
In conclusion, by using AC as the catalyst and water as solvent, a novel catalytic system was developed for the synthesis of pyrrolo [1, 2-a]quinoxalines. The reaction can be carried out smoothly under mild condition and the scope of amines and nitro compounds was extended widely. A variety of quinoxalines can be obtained with good to excellent yield. It is noteworthy that no transition metal, additive, oxidant and reductant were involved in this reaction. Only water was used as the solvent and the reactions can tolerate air. The reuse of AC catalyst could be achieved by a simple phase separation process without obvious loss of its catalytic activity. Studies on the application of this method and the detailed mechanism are in progress.
AcknowledgmentsWe are grateful for the financial support from the National Natural Science Foundation of China (Nos. 21432009, 21672200, 21472177, 21772185 and 21801233) and for the assistance of the product characterization from the Chemistry Experiment Teaching Center of University of Science and Technology of China. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20000000).
Appendix A. Supplementary dataSupplementary material related to this article canbe found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.007.
| [1] |
(a) S. Butini, R. Budriesi, M. Hamon, et al., J. Med. Chem. 52 (2009) 6946-6950; (b) L. Fan, N. Huang, R. Yang, et al., Lett. Drug Des. Discov. 9 (2012) 44-47; (c) P. Lin, D.B. Salunke, L. Chen, C. Sun, Org. Biomol. Chem. 9 (2011) 2925-2937; (d) J. Guillon, E. Mouray, S. Moreau, et al., Eur. J. Med. Chem. 46 (2011) 2310-2326; (e) D.S. Snyder, L. Tradtrantip, C. Yao, M.J. Kurth, A.S. Verkman, J. Med. Chem. 54 (2011) 5468-5477; (f) C. Bailly, S. Echepare, F. Gago, Anti-Cancer Drug Des. 14 (1999) 291-303; (g) J. Guillon, P. Dallemagne, B. Pfeiffer, et al., Eur. J. Med. Chem. 33 (1998) 293-308; (h) T.B. Nguyen, P. Retailleau, A. Al-Mourabit, Org. Lett. 15 (2013) 5238-5241; (i) T.B. Nguyen, J.L. Bescont, L. Ermolenko, A. Al-Mourabit, Org. Lett. 15 (2013) 6218-6221. |
| [2] |
(a) Q. Yuan, D. Ma, J. Org. Chem. 73 (2008) 5159-5162; (b) F. Zhao, L. Zhang, H. Liu, S. Zhou, H. liu, Beilstein J. Org. Chem. 9 (2013) 2463-2469; (c) J.T. Reeves, D.R. Fandrick, Z. Tan, et al., J. Org. Chem. 75 (2010) 992-994; (d) A.K. Verma, R.R. Jha, V.K. Sankar, et al., Eur. J. Org. Chem. 34 (2011) 6998-7010; (e) G. Liu, Y. Zhou, D. Lin, et al., ACS Comb. Sci. 13 (2011) 209-213; (f) J. Cuillon, I. Forfar, M.M. Matsuda, et al., Bioorg. Med. Chem. 15 (2007) 194-210. |
| [3] |
M.F. Pereira, V. Thiéry, Org. Lett. 14 (2012) 4754-4757. DOI:10.1021/ol302006b |
| [4] |
M. Ramamohan, R. Sridhar, K. Rageavendrarao, et al., Synlett 26 (2015) 1096-1100. DOI:10.1055/s-0034-1380347 |
| [5] |
(a) N. Mizuno, M. Misono, Chem. Rev. 98 (1998) 199-218; (b) R. Akiyama, S. Kobayashi, Chem. Rev. 109 (2009) 594-642; (c) S. Ikegami, H. Hamamoto, Chem. Rev. 109 (2009) 583-593; (d) M.J. Climent, A. Corma, S. Iborra, Chem. Rev. 111 (2011) 1072-1133 and references therein. |
| [6] |
(a) Y. Kawashita, N. Nakamichi, H. Kawabata, M. Hayashi, Org. Lett. 4 (2002) 3955-3957; (b) H. Sun, F.Z. Su, J. Ni, et al., Angew. Chem. Int. Ed. 48 (2009) 4390-4393; (c) N. Kan-nari, S. Okamura, S. Fujita, J. Ozaki, M. Arai, Adv. Synth. Catal. 352 (2010) 1476-1484; (d) L. He, Y. Qian, R. Ding, et al., ChemSusChem 5 (2012) 621-624; (e) D.T.D. Tang, K.D. Collins, F. Glorius, J. Am. Chem. Soc. 135 (2013) 7450-7453; (f) D.T.D. Tang, K.D. Collins, J.B. Ernst, F. Glorius, Angew. Chem. Int. Ed. 53 (2014) 1809-1813; (g) S. Fujita, H. Watanabe, A. Katagiri, H. Yoshida, M. Arai, J. Mol. Catal. A: Chem. 393 (2014) 257-262. |
| [7] |
(a) Z. Zhang, Z. Wang, J. Org. Chem. 71 (2006) 7485-7487; (b) J. Zhang, C. Yu, S. Wang, C. Wan, Z. Wang, Chem. Commun. 46 (2010) 5244-5246; (c) Y. Wang, G. Ouyang, J. Zhang, Z. Wang, Chem. Commun. 46 (2010) 7912-7914; (d) Y. Wang, D. Zhu, L. Tang, S. Wang, Z. Wang, Angew. Chem. Int. Ed. 50 (2011) 8917-8921; (e) H. Sun, Y. Zhang, F. Guo, Z. Zha, Z. Wang, J. Org. Chem. 77 (2012) 3563-3569; (f) H. Sun, Q. Hua, F. Guo, Z. Wang, W. Huang, Adv. Synth. Catal. 354 (2012) 569-573; (g) L. Tang, H. Sun, Y. Li, Z. Zha, Z. Wang, Green Chem. 14 (2012) 3423-3428; (h) L. Tang, X. Guo, Y. Li, et al., Chem. Commun. 49 (2013) 5213-5215; (i) X. Guo, L. Tang, Y. Yang, Z. Zha, Z. Wang, Green Chem. 16 (2014) 2443-2447; (j) L. Tang, X.F. Guo, Y. Yang, Z. Zha, Z. Wang, Chem. Commun. 50 (2014) 6145-6148; (k) L. Tang, Y. Yang, L. Wen, S. Zhang, Z. Zha, Z. Wang, Org. Chem. Front. 2 (2015) 114-118. |
| [8] |
V. Calvino-Casilda, A.J. López-Peinado, C.J. Durán-Valle, R.M. Martín-Aranda, Catal. Rev. Sci. Eng. 52 (2010) 325-380. DOI:10.1080/01614940.2010.498748 |
| [9] |
(a) Y. Kawashita, N. Nakamichi, H. Kawabata, M. Hayashi, Org. Lett. 5 (2003) 3713-3715; (b) Y. Kawashita, M. Hayashi, Molecules 14 (2009) 3073-3093; (c) Y. Kawashita, J. Yanagi, T. Fujii, Masahiko Hayashi, Bull. Chem. Soc. Jpn. 82 (2009) 482-488; (d) H. Watanabe, S. Asano, S.I. Fujita, H. Yoshida, M. Arai, ACS Catal. 5 (2015) 2886-2894. |
| [10] |
(a) Y. Yang, L. Tang, S. Zhang, et al., Green Chem. 16 (2014) 4106-4109; (b) L. Tang, Y. Yang, L. Wen, X. Yang, Z. Wang, Green Chem. 18 (2016) 1224-1228; (c) Y. Yang, S. Zhang, L. Tang, Y. Hu, Z. Zha, Z. Wang, Green Chem. 18 (2016) 2609-2613. |
| [11] |
(a) C.J. Li, Chem. Rev. 105 (2005) 3095-3166; (b) C.J. Li, L. Chen, Chem. Soc. Rev. 35 (2006) 68-82; (c) Y.R. Shen, V. Ostroverkhov, Chem. Rev. 106 (2006) 1140-1154; (d) C.I. Herrerías, X. Yao, Z. Li, C.J. Li, Chem. Rev. 107 (2007) 2546-2562; (e) M. Simon, C.J. Li, Chem. Soc. Rev. 41 (2012) 1415-1427; (f) M.B. Gawande, V.D.B. Bonifácio, R. Luque, P.S. Branco, R.S. Varma, Chem. Soc. Rev. 42 (2013) 5522-5551. |
 2019, Vol. 30
2019, Vol. 30