b Ophthalmic Laboratory and Department of Ophthalmology, State Key Laboratory and Collaborative Innovation Center of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041, China
Gold-based nanoparticles have been highlighted in recent years as one of the most promising nano-biomaterials in cancer diagnosis and therapy due to their unique optical properties and stability [1]. The uncovering of localized surface plasmon resonance (LSPR) effect has stimulated the invention of goldbased nanoparticles with controllable shapes and dimensions [2, 3]. It also found out that the shapes and sizes have critical impact onto the LSPR effects of gold-based nanoparticles, for example, the gold nanorods (GNRs), gold nanocages (GNCs), gold nanostars (GNSs) not only have high efficiency in photo-thermal conversion, but also can act as signal enhancers [4]. The further development and applications of gold-based nanoparticles in biomedical field have been gaining enormous attentions [5].
Beyond GNRs, GNCs, or GNSs, etc., gold nanoprisms (GNPs) is another kind of gold-based nanostructure. Because of its triangle structure, GNPs exhibit some differently optical performance, especially serve as signal enhancer in surface-enhanced resonant Raman scattering (SERRS) imaging, which is also an interesting features of gold-based nanoparticles. Gold nanoparticles can be easily functionalized with positively charged hydrophobic NIR dyes to form SERRS nanoparticles [6-8]. Comparing with the commercial SERRS imaging agents which are choosing gold nanospheres as the core for signal enhancement, GNPs are offering higher SERRS effects, while can be ascribed to the presence of sharp tips and rich hot spots when compared to the spheres [9]. Furthermore, GNPs also can be served as the agent of photothermal conversion for photothermal therapy (PTT) [10, 11]. They have strong absorption in 650–700 nm light region [12, 13]. By carefully controlling, the adsorption peak of GNPs can be adjusted to ~660 nm, which can be converted the 660 nm laser to generate hyperthermia, thus, realize PTT. However, the lack of tumortargeting ligand modification has impeded the further applications of GNPs in tumor diagnosis and therapy by maintaining the SERRS and PTT performance of GNPs.
To resolve this barrier, we planned to use a tumor-homing peptide, LyP-1, to enhance the enrichment of GNPs in tumor, and then to enhance the GNPs-mediated PTT. LyP-1 bound specifically to p32 (also known as gC1qR) receptor which was overexpressed on the cell surface of tumor lymphatics and solid tumor, often in hypoxic areas deep in the tumor tissue upregulated in the endothelium during angiogenesis [14]. Hence, LyP-1 conjugated nanocarriers showed good selectivity in lymph nodes metastatic tumors and breast cancer [15-17]. And the existing of thiol group containing in the sequence of LyP-1 also provides an ideal group to react with GNPs. What is more important, in order to avoid the dye leaching and resist aggregation in the physiological environment, thiolated polyethylene glycol (SH-PEG) was used as shelter. And a near infrared (NIR) dye, IR780, was used as model dye for SERRS imaging [18, 19]. The NIR dye IR780 bound covalently on the gold surface via nitro groups, and the further surface PEGylated and functionalized with linear LyP-1 peptide (CGNKRTRGC, with two free sulfhydryl groups), rendering the GNPs/IR780-LyP-1 as a multifunctional platform for SERS imaging and targeting photothermal therapy for breast cancer (Scheme 1).

|
Download:
|
| Scheme 1. Schematic illustration of tumor targeting therapy by the peptide conjugated-GNPs nanosystem. The NIR dye IR780 not only enabled the GNPs-based nanosystem as SERRS nanoparticles for Raman-encoded molecular imaging, but also enhanced the plasmonic photothermal property by laser irradiation. Meanwhile, the GNPs/IR780-Lyp-1 by introduction of tumor homing peptide segment of LyP-1, which presents high affinity to p32 protein, demonstrate the increased enrichment in tumor region and enhanced photothermal therapy efficacy. | |
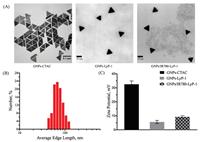
The morphology of GNPs, GNPs-LyP-1 and the GNPs/IR780-LyP-1 were characterized by TEM. The homogeneous triangle shape of nanoprisms with average edge length of 57.5 ± 9.3 nm had been observed for GNPs, and no obvious aggregation or morphological change was observed after the surface modification (Figs. 1A and B). The zeta potential of CTAC stabilized GNPs was +32.5 ± 2.35 mV, whereas GNPs-LyP-1 and GNPs/IR780-LyP-1 was 5.60 ± 1.11 mV and 9.27 ±0.86 mV, respectively, which also indicated the SH-PEG, LyP-1 peptide and positive IR780 were attached to the surface of GNPs (Fig. 1C).
|
Download:
|
| Fig. 1. (A) TEM imaging of GNPs-CTAC, GNPs-LyP-1 and GNPs/IR780-LyP-1 (Scale bar: 0.1 μm and 50 nm); (B) The edge length distribution of GNPs; (C) Zeta potential of GNPsCTAC, GNPs-LyP-1 and GNPs/IR780-LyP-1. | |
The UV–vis spectra of GNPs are depicted in Fig. 2A. The distinct peaks around 600 nm can be observed with a slightly blue-shifted for the LyP-1-conjugated and PEGylated GNPs, and the IR780 bound resulted in a red-shifted. The SERRS spectra generated by the GNPs-LyP-1 and GNPs/IR780-LyP-1 were measured at the Au concentrations of 10-4 mol/L, at an excitation wavelength of 785 nm (Fig. 2B). The GNPs-LyP-1 solution exhibited a very low signal-to-noise ratio, whereas the GNPs/IR780-LyP-1 presented enhanced Raman signal strength. The background is related to IR780 fluorescence, probably originated from the free molecules in solution. The enhanced Raman spectra signal suggested that the gold nanoprisms system is a promising candidate for Raman encoded molecular imaging of tissues besides other nanostars or nanorods-based SERS nanoparticles [7, 20, 21].

|
Download:
|
| Fig. 2. (A) Normalized UV–vis spectra of GNPs-CTAC, GNPs-LyP-1 and GNPs /IR780-LyP-1 solution; (B) The Raman scattering spectra of saline, GNPs-LyP-1 and GNPs/IR780-LyP-1 solution excited at 785 nm; (C) Photothermal imaging of different samples with 660 nm laser irradiation at a fixed concentration of Au (50 μg/mL) (laser power: 1.0 W/cm2); (D) Temperatures elevation of samples based on IR thermal imaging data. | |
The photothermal conversion of GNPs-CTAC, GNPs-LyP-1 and GNPs/IR780-LyP-1 were systematically investigated by monitoring temperature increase while exposed to a 660 nm laser (1 W/cm2). As depicted in Figs. 2C and D, the temperature of the GNPs/IR780-LyP-1 solution (with the Au concentration of 50 μg/mL) reached 44.9 ℃ with laser irradiation as short as 3 min, and its final temperature was dramatically increased from 25 ℃ to 51 ℃ after 5 min irradiation, which was higher than those of GNPs-LyP-1 and GNPs-CTAC did. In comparison, the temperature of saline showed no significant change when exposed to the same laser. The results demonstrated that GNPs/IR780-LyP-1 could significantly generate heat when exciting with 660 nm laser and be utilized as the promising agent for PTT.
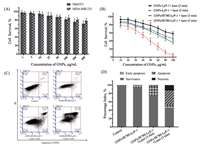
As the biomedical materials, the biosafety is critically important for their further application. The surfactant CTAC utilized for shape controlling and colloid stabilizing of GNPs is responsible for the cytotoxicity, and the PEG modification is reported as a common method to reduce cytotoxicity from cationic surfactant stabilized gold nanoparticles [22, 23]. So, the cytotoxicity of GNPs/IR780-Lyp-1 was investigated on both NIH3T3 and MDA-MB-231 cells by MTT assay, as shown in Fig. 3A. The cell survival was nearly 80% even after incubation with GNPs /IR780-Lyp-1 at the mean concentration of 200 μg/mL, indicating the biocompatibility at the cellular level. When a 660 nm laser irradiation introduced, the different treated MDA-MB-231 cells presented cell growth inhibition, and the GNPs/IR780-Lyp-1 with 5 min laser exposed showed better antitumor performance (Fig. 3B). To further evaluate the thermotherapy effect mediated by GNPs/IR780-Lyp-1 on the MDA-MB-231 cell growth inhibition, cell apoptosis and necrosis were quantified by cell apoptosis assay (Fig. 3C). The data analysis showed that treatment with GNPs/IR780-Lyp-1 plus 660 nm laser irradiation significantly increased cell apoptosis, when compared with no laser irradiation. Notably, the PTT for 5 min resulted in 42% of cell apoptosis and 12.8% of cell necrosis, while only 25.3% of apoptotic cells was detected by the treatment with laser irradiation for 3 min (Fig. 3D). These data suggest that photothermal-induced apoptosis of MDA-MB-231 cells was time-dependent.
|
Download:
|
| Fig. 3. (A) The cytotoxicity of GNPs/IR780-Lyp-1 on NIH3T3 or MDA-MB-231 cell lines without laser; (B) MTT assays for GNPs-Lyp-1 and GNPs/IR780-Lyp-1 on MDA-MB-231 cell line with laser irradiation; (C) Apoptosis assay of MDA-MB-231 cells after 8 h incubation with GNPs/IR780-Lyp-1 plus different laser irradiation manners; (D) Percentage of early apoptotic, apoptotic, necrotic, and survivance cells according to the data of apoptosis assay. | |
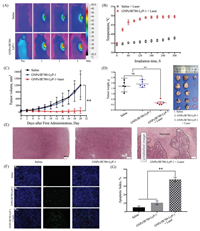
Based on the in vitro photothermal conversion results, we have investigated therapeutically relevant temperature increase in vivo. As shown in Fig. 4A, after 8 h of each sample administration, the mice were treated with 660 nm laser irradiation for 5 min. The in vivo photothermal imaging presented that the temperature of the tumor region which was treated with GNPs/IR780-Lyp-1 rapidly increased to 46.6 ℃ in 1 min, and exhibited a significantly temperature increase of 14.7 ℃ after 5 min irradiation (Fig. 4B). In contrast, animals treated with saline presented no obvious temperature rise as the irradiation time prolonged, which was further confirmed that the LyP-1 modification could enhance the enrichment of nanosystem in tumor region [24, 25].
|
Download:
|
| Fig. 4. (A) Photothermal images of tumor-bearing mice treated with saline and GNPs/IR780-Lyp-1 under the irradiation of 660 nm laser (power: 1 W/cm2); (B) Relationships of photothermal conversion efficiency with different treatment in vivo; (C) The tumor volume curves for each group with different treatments; (D) The weights and the photographs of subcutaneous tumors after different treatments. (E) H & E staining of tumor tissue slices after different treatments: (1) saline, (2) GNPs/IR780-Lyp-1, (3) GNPs/ IR780-Lyp-1 + 660 nm laser (1 W/m2 for 5 min). Scale bar represents 200 μm; (F) The TUNEL immunofluorescence staining of tumor slices with different treatment in MDAMB-231 model. Scale bar represents 50 μm; (G) The quantitative analysis of TUNEL immunofluorescence in each group. (All data are expressed as means ± SD (n = 5), and **P < 0.01 was considered as significant difference). | |
Herein, the in vivo tumor PTT efficacy of the GNPs/IR780-Lyp-1 was evaluated in MDA-MB-231 tumor models, and the tumor volume of each mouse was recorded with a one-day interval. As the subsequent antitumor results showed in Fig. 4C, no inhibition of tumor growth was observed in the group administrated with GNPs/IR780-Lyp-1 without laser irradiation. Compared to the control group (saline) and GNPs/IR780-Lyp-1 without laser treated group, the tumor growth was significantly inhibited after treatment of the same nanosystem when 660 nm laser irradiation utilized, which contributed to the PTT effects. Notably, the tumors of laser treated group were totally eliminated in 2 of the 5 mice. Furthermore, the tumor weight of mice showed significant decrease between the PTT treated group and control group (Fig. 4D). These results also demonstrated that the nanosystem of GNPs/IR780-Lyp-1 could accumulate at the tumor region and perform better anticancer effect when PTT introduced.
The PTT-enhanced tumor growth inhibition of GNPs/IR780-Lyp-1 was further proved by the H & E staining and immunofluorescence analysis for tumor tissues. As shown in Fig. 4E, no significant necrosis in tumors was observed in the control group and GNPs/ IR780-Lyp-1 treated only group. In contrast, a large necrotic area was observed after 2 days of GNPs/IR780-Lyp-1 treatment by PTT. The PTT induced hyperthermia nearly 50 ℃ which would cause acute hemorrhage and tumor necrosis. Moreover, based on the immunofluorescence analysis of the tumor slides with different treatments, the apoptosis of tumor cells was clearly detected after PTT treatments (Figs. 4F and G). The result further indicated that tumor cell proliferation was inhibited by the tumor homingenhanced PTT, which was correspondent with the results of cellular apoptosis assay.
In summary, the tumor-homing peptide LyP-1 was chosen as the targeting moiety for the gold nanoprisms (GNPs) functionalization in this study. Moreover, because of the existence of NIR dye IR780, GNPs/IR780-LyP-1exhibited the surface-enhanced resonant Raman scattering property and plasmonic photothermal property which delivered a therapeutic heating by 660 nm laser irradiation. As compared to GNPs-LyP-1, the GNPs/IR780-LyP-1 presented significantly increased of photothermal conversion in vitro. And the tumor growth inhibition was further enhanced by the LyP-1 peptide mediated tumor targeting photothermal therapeutic efficacy after laser irradiation. All the results demonstrate that the GNPs/IR780-LyP-1 prepared in this study can be served as a promising SERRS nanoparticles for Raman-encoded molecular imaging-guided enhanced photothermal therapy.
AcknowledgmentsThis work was financially supported by the Application Fundamental Research Foundation of Sichuan Province Science and Technology Department, China (No. 2016JY0157), the State Sponsored Scholarship for Visiting Scholar from China Scholarship Council, the Outstanding Science and Technology Projects for Returned Overseas Chinese Scholars, Sichuan Province, China, the National Natural Science Foundation of China (No. 31600811), the National Training Program of Innovation and Entrepreneurship for Undergraduates (No. 201813705025).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.02.019.
| [1] |
W. Zhou, X. Gao, D.B. Liu, X.Y. Chen, Chem. Rev. 115 (2015) 10575-10636. DOI:10.1021/acs.chemrev.5b00100 |
| [2] |
C. Zhou, J.Q. Huang, Q. Yang, et al., Colloids Surf. B Biointerfaces 171 (2018) 17-23. DOI:10.1016/j.colsurfb.2018.07.002 |
| [3] |
Q. Yang, J.R. Peng, Y. Xiao, et al., ACS Appl. Mater. Interfaces 10 (2018) 150-164. DOI:10.1021/acsami.7b14705 |
| [4] |
L.A. Lane, R. Xue, S. Nie. Curr. Opin. Chem. Biol. 45 (2018) 95-103. DOI:10.1016/j.cbpa.2018.03.015 |
| [5] |
H.D. Cui, D.H. Hu, J.N. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1391-1398. DOI:10.1016/j.cclet.2016.12.038 |
| [6] |
H. Lei, Y. Hu, G. Li, Chin. Chem. Lett. 29 (2018) 509-512. DOI:10.1016/j.cclet.2017.08.012 |
| [7] |
Y. Wang, Q. Yang, S. Kang, M.A. Wall, J.T.C. Liu, J. Biomed. Opt. 23 (2018) 1-8. |
| [8] |
M.W. Li, Y.Y. Qiu, C.C. Fan, et al., Acta Pharma. Sin. B 8 (2018) 381-389. DOI:10.1016/j.apsb.2018.01.010 |
| [9] |
H. Yuan, Y. Liu, A.M. Fales, et al., Anal. Chem. 85 (2013) 208-212. DOI:10.1021/ac302510g |
| [10] |
Pérez-Hernández M., del Pino P., S.G. Mitchell, et al., ACS Nano 9 (2015) 52-61. DOI:10.1021/nn505468v |
| [11] |
S.Q. Yang, L.Z. Zhou, Y. Su, R. Zhang, C.M. Dong, Chin. Chem. Lett. 30 (2019) 187-191. DOI:10.1016/j.cclet.2018.02.015 |
| [12] |
Y. Zhao, W. Liu, Y. Tian, et al., ACS Appl. Mater. Interfaces 10 (2018) 16992-17003. DOI:10.1021/acsami.7b19013 |
| [13] |
C. Kuttner, M. Mayer, M. Dulle, et al., ACS Appl. Mater. Interfaces 10 (2018) 11152-11163. DOI:10.1021/acsami.7b19081 |
| [14] |
V. Fogal, L. Zhang, S. Krajewski, E. Ruoslahti, Cancer Res. 68 (2008) 7210-7218. DOI:10.1158/0008-5472.CAN-07-6752 |
| [15] |
Z.Q. Yan, F. Wang, Z.Y. Wen, et al., J. Control. Release 157 (2012) 118-125. DOI:10.1016/j.jconrel.2011.07.034 |
| [16] |
L. Paasonen, S. Sharma, G.B. Braun, et al., ChemBioChem 17 (2016) 570-575. DOI:10.1002/cbic.v17.7 |
| [17] |
Y. Jiang, S. Liu, Y. Zhang, et al., Biomaterials 115 (2017) 9-18. DOI:10.1016/j.biomaterials.2016.11.006 |
| [18] |
S. Harmsen, M.A. Wall, R. Huang, M.F. Kircher, Nat. Protoc. 12 (2017) 1400. DOI:10.1038/nprot.2017.031 |
| [19] |
Nagy-Simon T., M. Potara, A.M. Craciun, E. Licarete, S. Astilean, J. Colloid Interf. Sci. 517 (2018) 239-250. DOI:10.1016/j.jcis.2018.02.007 |
| [20] |
F.R. Tian, J. Conde, C.C. Bao, et al., Biomaterials 106 (2016) 87-97. DOI:10.1016/j.biomaterials.2016.08.014 |
| [21] |
J.V. Jokerst, A.J. Cole, Van de Sompel D., S.S. Gambhir, ACS Nano 6 (2012) 10366-10377. DOI:10.1021/nn304347g |
| [22] |
Y.P. Jia, B.Y. Ma, X.W. Wei, Z.Y. Qian, Chin. Chem. Lett. 28 (2017) 691-702. DOI:10.1016/j.cclet.2017.01.021 |
| [23] |
X.C. Tang, L.W. Tan, K. Shi, et al., Acta Pharma. Sin. B 8 (2018) 587-601. DOI:10.1016/j.apsb.2018.05.011 |
| [24] |
Escudero-Francos M.A., V. Cepas, González-Menéndez P., et al., J. Biomed. Nanotechnol. 13 (2017) 167-179. DOI:10.1166/jbn.2017.2344 |
| [25] |
F.C. Tian, F.Z. Fatima, J.N. Qiao, et al., Acta Biomater. 75 (2018) 398-412. DOI:10.1016/j.actbio.2018.05.050 |
 2019, Vol. 30
2019, Vol. 30 

