The construction of one-, two-, and three-dimensional (1D, 2D, and 3D) coordination polymers has resulted in porous materials with various kinds of structures and potential applications in different fields [1-3]. Among which, multi-layers of (4, 4) nets [4-6] and diamondoid network topologies [7, 8] stand out because of their desirable and predictable conformations in possessing large cavities or channels and potential structural transformations subject to variations in solvents, counter anions or guest species. The study of relationship between interpenetrated and noninterpenetrated frameworks between (4, 4)-sql grids and multiinterpenetrated diamondoid (dia) networks provides an approach to better understanding the mechanism of supramolecular selfassembly [9, 10]. In general, (4, 4)-sql network can be constructed from a square-planar nodal point propagated in four directions on a plane, while dia- framework can be fabricated by propagating a tetrahedral (Td) nodal point in all four directions in three dimensions. Furthermore, the different geometry parameters involved in the square or tetrahedral coordination nodes will determine the shapes and dimensions of the cavities inhabited in the grids or frameworks. Other ways known from literatures to be able to tune the interpenetration and cavity size in coordination polymers include preparation of charged networks, introduction of strong interactions between the networks, clathration of guest molecules, utilization of large building blocks, design of inherently porous network topology, and so on [11].
We have reported multidentate ligands comprising a rigid central arene group as spacer and two or more flexible donor pendants, such as pyridyl (Py), imidazolyl (Im) and benzimidazolyl (Bim) groups, as arms to assemble discrete cyclic or cage structures as well as infinite polymeric structures [12, 13], which can take different coordination modes in assembling targeted structures. In this paper, we report the assembly of the semirigid "arm-spacerarm" type ligand, N, N'-bis(4-pyridylmethyl)naphthalene diimide (4-pmntd, Scheme S1 in Supporting information) with different transition metal salts, giving five non-interpenetrated (4, 4) layers or multi-interpenetrated diamondoid coordination polymers. Tuning of crystal structures is achieved by the different conformations and spatially extending geometries adopted by the ligand and the introduction of pillar-like anions or guest molecules.
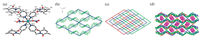
Complex 1 ({[Cu(4-pmntd)2(NO3)2]·2CHCl3}n) crystallizes in the monoclinic system with the space group P2(1)/c. The Cu(Ⅱ) centers are hexa-coordinated, as shown in Fig. 1a. This hexacoordinated CuN4O2 environment is composed of four nitrogen atoms from four independent 4-pmntd ligands and two oxygen atoms from two NO3-. Due to Jahn-Teller effects, the axial O atoms have longer distances from Cu center of about 2.58 Å, while the distances from equatorial N atoms are shorter, being 2.01-2.03 Å. In this complex, L adopts Z-mode configuration, with the two semiflexible pyridyl arms bending to the opposite sides of the rigid 1, 4, 5, 8-naphthalene diimide spacer with an average distortion angle of 64.8°, and acts as a bridge between two Cu(Ⅱ) ion. In another respect, each Cu(Ⅱ) is connected to four ligands, which are stretching in a plane, resulting in two-dimensional sheets in ab plane having bat-like M-L connection mode, which could be represented by (4, 4)-sql topology using Cu(Ⅱ) centers as the nodes (Fig. 1b). The size of the M4L4 square is about 1.9 × 1.9 nm2. Layers and layers are further stacked in an edge-to-edge way to result in non-interpenetrated 3D framework (Fig. 1c), in which the solvated chloroform molecules are existed, showing no obvious intermolecular interactions with the host (Fig. 1d). The potential solvent volume is up to 35.3% as estimated by Platon [14].
|
Download:
|
| Fig. 1. The crystal structures of complex 1. (a) Coordination environment of Cu(Ⅱ) ion and atom labeling scheme, (b) the bat-like M-L connection mode and (4, 4) net represented, (c) edge-to-edge packing of (4, 4) layers, and (d) solvent molecules existed between the layers. | |
Complex 2 ({[Cu(4-pmntd)2(NO3)2]·3C7H8}n) has the coordination environment resembling that in 1, in which the Cu(Ⅱ) center is hexa-coordinated by four N atoms from four independent 4-pmntd ligands and two oxygen atoms from two NO3-, as shown in Fig. S1a (Supporting information). The Cu-N and Cu-O bond lengths are averaged 2.334(5) Å and 2.352(5) Å, respectively, and the distortion angles between four pyridyl rings to naphthalene diimide spacer are ranged from 64° to 85°. The bat-like M-L connected twodimensional sheets and represented (4, 4) topological nets in complex 2 are also similar to that in complex 1 (Fig. S1b in Supporting information), which are stacked in an edge-to-edge offset way, giving rise to an infinite 3D framework. The layers are sustained by the hydrogen bonds formed between the O atoms on NO3- and naphthalene diimide from one layer, and the CH- groups on naphthalene diimide from another layer, with distances from 3.2 Å to 3.5 Å, as shown in Fig. S1c (Supporting information). Furthermore, guest toluene molecules are hosted between the layers, through π…π interactions (with centroid distance of 3.4 Å) and C-H…O bonds (Figs. S1d and e in Supporting information). The potential solvents volume in complex 2 is 35.3%.
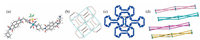
In complex 3 ({[Cu(4-pmntd)2(CF3SO3)(H2O)]·CF3SO3·H2O· CH3OH}n), L adopts the U-mode configuration to connect the Cu (Ⅱ) metal centers, which are hexa-coordinated by four nitrogen atoms from four independent 4-pmntd ligands, one oxygen atom from CF3SO3- and one oxygen atom from H2O, as shown in Fig. 2a. Due to the different conformation adopted by the ligand, a dumbbell-like M-L connected pattern is formed, and can also be represented by (4, 4) topological grids when metal centers are used as nodes (Fig. 2b). The M4L4 rhombic grid has an average edge length of 15.0 Å, in which another non-coordinated CF3SO3- ion is located. The torsion angles between four pyridyl groups and naphthalene diimide spacer are ranged from 68° to 78°. In the dumbbell-like connections, two opposite ligands share a strong π…π interaction between the naphthalene diimide planes (with centroid distance of 3.338 Å) to lower the energy, as shown in Fig. 2c. Layers and layers are further stacked in an offset style into 3D frameworks, stabilized by C-H…O bonds formed between the O atoms on the ligand from one layer and the CH- groups on naphthalene diimide (3.17-3.22 Å), pyridyl groups (3.31 Å) and methylene groups (3.46 Å) from the ligand in an adjacent layer (Fig. 2d and Fig. S2 in Supporting information). Solvated water and methanol molecules are located between the layers, also through the connection of C-H…O bonds (3.46 Å). Compared to complexes 1 and 2, the U-mode of the ligand in complex 3 results in smaller inner cavities between the grids and denser packing modes among the layers, so the potential solvent volume in the complex is only 12.5%.
|
Download:
|
| Fig. 2. The crystal structures of complex 3. (a) Coordination environment of Cu(Ⅱ) ion and atom labeling scheme, (b) the dumbbell-like M-L connection mode and (4, 4) net represented, (c) π…π interactions between the dumbbell-like M-L connection, (d) edge-to-edge packed layers. | |
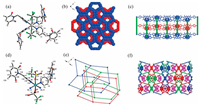
Complex 4 {([Cd(4-pmntd)2]n·nSiF6·x(CH3OH)·y(CHCl3)} is crystallized in tetragonal crystal system with P4/n space group, which also uses the U-mode conformation of the ligand and forms dumbbell-like M-L connection patterns in which the Cd(Ⅱ) centers are hexa-coordinated by four nitrogen atoms from four independent 4-pmntd ligands, and two fluorine atoms from SiF62- anions, as shown in Fig. 3a. The axial F atoms have longer distances from Cd center of about 2.44 Å (Cd(1)-F(3)) and 2.40 Å (Cd(1)-F(6)), while the distances from equatorial N atoms are shorter, being 2.29 Å, due to Jahn-Teller effects. Different from the close packing in complex 3, the layers in complex 4 comprised by dumbbell-like M-L connectors on ab plane which can be represented by (4, 4) topology are supported by pillar-like SiF62- anions through strong Si-F coordination bonds along c direction, resulting in spacious square channels (dimensions: 3 × 3 Å2) between the layers (Figs. 3b and c). Helpfully, the potential solvent volume is greatly enlarged to 50.8%, which are occupied by large quantities of solvent molecules.
|
Download:
|
| Fig. 3. The crystal structures of complexes 4 (a–c) and 5 (d–f). (a) Coordination environment of Cd(Ⅱ) ion and atom labeling scheme, (b) dumbbell-like M-L connection mode and layer packing (solvents and anions omitted), and (c) schematic view of adjacent layers supported by SiF6- anions (represented by green pillars). (d) Coordination environment of Zn(Ⅱ) ion and atom labeling scheme, (e) 3-fold interpenetrating dia-network, and (f) channels along c axis and occupied solvents in space-filling mode. | |
Complex 5 {[Zn(4-pmntd)2(CF3SO3)2]n·χ(solvent)} crystallizes in the monoclinic crystal system with the space group C2/c. The Zn (Ⅱ) centers are hexa-coordinated, forming ZnN4O2 environment, which is composed of four nitrogen atoms from four independent 4-pmntd ligands, and two oxygen atoms from CF3SO3-, as shown in Fig. 3d. In this complex, the ligands take Z-mode configuration to connect the Zn(Ⅱ) centers into 3D diamond-like (dia) networks. Due to the spacious nature of the net, there are in fact three identical complementary networks, interpenetrating to each other, as shown in Fig. 3e. If the guest molecules are omitted, channels with an opening size of 4 × 3 Å2 are clearly visible along the crystallographic c axis as shown in Fig. 3f, and the potential solvent volume is up to 28.8%.
Form the structures discussed in complexes 1-5, we can find that the semirigid ligand 4-pmntd, as depicted in Scheme 1, may take on U or Z conformations to participate in coordination with metal ions. Since the energy barrier required for interconversion between these two different conformations is expectably small, the ligand can display specific conformation mainly adaptive to the metal center's coordination environment subjected to influence from counter anions in the assembly process. Generallly, the ligand prefers to connect the metal centers in Z-mode, as the cases in complexes 1, 2 and 5, which will result in either relatively loose packing of non-interpenetrated bat-like type (4, 4) nets or lead to multi-interpenetrated diamondoid networks. In contrast, in some special conditions, such as introducing large CF3SO3- anions into the coordination sphere or using pillar-like anions like SiF6- in complexes 3 and 4, U conformation of the ligand can be formed, which will both result in non-interpenetrated packing of dumbbell-like type (4, 4) nets but with two opposite tendencies in modifying cavities. One will be densely packed between layers, leaving limited cavities in the frameworks (complex 3, 12.5%), while the other will greatly broaden the inner channels by the upholding of SiF6- pillars (complex 4, 50.8%).

|
Download:
|
| Scheme 1. Structural tuning modes in complexes 1–5: Conformations of ligand, stretching modes of 4-connecting metal centers, and "secondary building process" of layer structures into 3D spacious frameworks. | |
Furthermore, although all the metal centers in complexes 1-5 adopt six-coordinated geometry, the spatially stretching mode of the four ligands around the metal center will tremendously influence the interpenetrating tendency of the building units and determine the cavities shape and size in the complexes as well. If we use a geometry figure to represent the 4-connecting M-L units in complexes 1 and 5, we can clearly see that in complex 1, the geometry is basically planar, therefore, 2D non-interpenetrated (4, 4) topological nets could be formed. Comparatively, in complex 5, the four ligands stretch in three dimensions, resulting in a tetrahedron connecting node, so 3D diamondoid topological network will come into being with multi-interpenetration (Scheme 1).
Comparison between complexes 4 and 5 shows that although they both contain U-mode of the ligand and dumbbell-like M-L connecting sheets represented by (4, 4) topology, the potential solvent volumes of the two complexes are greatly different, with the former one only 12.5% and the latter one up to 50.8%, due to the supporting effects of SiF6- among layers in complex 5. As shown in Scheme 1, the assembly of layers through the connection of strong Si-F coordination bonds using SiF6- as supplementary coordination connectors can be called a "secondary building process", in which secondary ligands or anions can be used to uphold compact layers into spacious 3D frameworks with expanded channels [15]. Using this method, pyrazine or 1, 4-diazabicyclo[2.2.2] octane which are with the similar length to SiF6- might also have the ability to organize this series of complexes into frameworks with large inner channels, and the study is undergoing in our group.
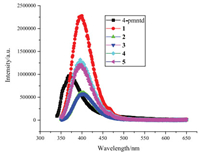
Priliminary luminescent properties of the ligand and complexes 1-5 are tested and shown in Fig. 4. It can be seen that the ligand 4- pmntd having large conjugated system can emit strong blue luminescence (371 nm) at the excitation of 330 nm light. The emitting peaks in complexes 1-5 are basically ligand-centered, showing in similar positions at ~400 nm, and with ~30 nm redshift compared with the ligand due to the coordination effect of the metal ions. The nanosecond lifetime (ranged from 2.3 ns to 5.0 ns) obtained for the complexes in the time decay experiment also proves the assignment of the luminescence to be ligand-centered.
|
Download:
|
| Fig. 4. Emission spectra of the ligand and complexes 1–5. | |
In summary, a semirigid ditopic ligand with two relatively flexible pyridyl arms and its Cu(Ⅱ), Cd(Ⅱ), Zn(Ⅱ) coordination polymers have been synthesized and structurally characterized. When using different metal centers, anions and solvents, the ligand can take on Z or U-mode conformations to assemble fourconnecting metal nodes and stretch either in a plane or in three dimensions, resulting in 2D bat-like or dumbbell-like (4, 4)-sql topological or 3D dia- type building units. Further non-interpenetrated packing among layers or multi-interpenetration of complementary networks will bring different shapes and sizes to the inner cavities. Especially, introduction of pillar anions will lead to "secondary building process" among layers of the coordination polymers, and have great effects to tune the interpenetration and cavity size of the complexes.
AcknowledgmentsThis work was supportedbyNSFC (Nos.21771197, 21720102007, 21821003), Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (No. 2017BT01C161), and FRF for the Central Universities.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2019.02.001.
| [1] |
(a) W.P. Lustig, S. Mukherjee, N.D. Rudd, et al., Chem. Soc. Rev. 46 (2017) 3242-3285; (b) Y. Zhang, S. Yuan, G. Day, et al., Coord. Chem. Rev. 354 (2018) 28-45; (c) M. Pan, K. Wu, J.H. Zhang, C.Y. Su, Coord. Chem. Rev. 378 (2017) 333-349; (d) M. Pan, W.M. Liao, S.Y. Yin, S.S. Sun, C.Y. Su, Chem. Rev. 118 (2018) 8889-8935. |
| [2] |
(a) M. Yoon, R. Srirambalaji, K. Kim, Chem. Rev. 112 (2012) 1196-1231; (b) L.Q. Ma, C. Abney, W.B. Lin, Chem. Soc. Rev. 38 (2009) 1248-1256; (c) K. Sumida, D.L. Rogow, J.A. Mason, et al., Chem. Rev. 112 (2012) 724-781.
|
| [3] |
(a) A.K. Chaudhari, H.J. Kim, I. Han, J.C. Tan, Adv. Mater. 29 (2017) 1701463; (b) L. Chen, J.W. Ye, H.P. Wang, et al., Nat. Commun. 8 (2017) 15985; (c) W.M. Liao, J.H. Zhang, S.Y. Yin, et al., Nat. Commun. 9 (2018) 2401. |
| [4] |
(a) S.Y. Hao, S.X. Hou, K.Van Hecke, G.H. Cui, Dalton Trans. 46 (2017) 1951-1964; (b) T.K. Maji, K. Uemura, H. C. Chang, R.Matsuda, S. Kitagawa, Angew.Chem. Int. Ed. 43 (2004) 3269-3272. |
| [5] |
(a) R. Liang, Y.K. Guo, Y.T. Wang, X.P. Xuan, Inorg. Chim. Acta 471 (2018) 50-56; (b) K. Takaoka, M. Kawano, M. Tominaga, M. Fujita, Angew. Chem. Int. Ed. 44 (2005) 2151-2154. |
| [6] |
(a) S.R. Zheng, S.Y. Yin, M. Pan, et al., Inorg. Chem. Commun. 55 (2015) 116-119; (b) M. Pan, B.B. Du, Y.X. Zhu, et al., Chem.-Eur. J. 22 (2016) 2440-2451; (c) S.L. Cai, M. Pan, S.R. Zheng, et al., CrystEngComm 14 (2012) 2308-2315. |
| [7] |
(a) L. Carlucci, G. Ciani, D.M. Proserpio, S. Rizzato, Chem.-Eur. J. 8 (2002) 1519-1526; (b) P. Mondal, B. Dey, S. Roy, et al., Cryst. Growth Des. 18 (2018) 6211-6220. |
| [8] |
a) S. Liu, M. Guo, Y. Sun, et al., Inorg. Chim. Acta 474 (2018) 73-80; (b) R.B. Lin, S. Xiang, B. Li, et al., Isreal J. Chem. 58 (2018) 949-961. |
| [9] |
S. Hu, K.H. He, M.H. Zeng, H.H. Zou, Y.M. Jiang, Inorg. Chem. 47 (2008) 5218-5224. DOI:10.1021/ic800050u |
| [10] |
H.D. Mai, I. Lee, S. Lee, H. Yoo, Eur. J. Inorg. Chem. 31 (2017) 3736-3743. |
| [11] |
(a) Z. Han, W. Shi, P. Cheng, Chin. Chem. Lett. 29 (2018) 819-822; (b) T. Xia, J. Wang, K. Jiang, et al., Chin. Chem. Lett. 29 (2018) 861-864; (c) W.H. Wang, Q. Gao, A.L. Li, et al., Chin. Chem. Lett. 29 (2018) 336-338. |
| [12] |
(a) M. Pan, Y.X. Zhu, K. Wu, et al., Angew. Chem. Int. Ed. 56 (2017) 14582-14586; (b) W.M. Liao, J.H. Zhang, Z. Wang, et al., J. Mater. Chem. A 6 (2018) 11337-11345. |
| [13] |
(a) S.R. Zheng, M. Pan, K. Wu, et al., Cryst. Growth Des. 15 (2015) 625-634; (b) Y.X. Zhu, Z.W. Wei, M. Pan, et al., Dalton Trans. 45 (2016) 943-950; (c) H.Y. Deng, J.R. He, M. Pan, L. Li, C.Y. Su, CrystEngComm 11 (2009) 909-917; (d) H.J. Yu, Z.M. Liu, S.Y. Yin, et al., Inorg. Chem. Commun. 86 (2017) 223-226; (e) H.J. Yu, Z.M. Liu, M. Pan, et al., Eur. J. Inorg. Chem. 1 (2018) 80-85. |
| [14] |
A.L. Spek, J. Appl, Cryst. 36 (2003) 7-13. DOI:10.1107/S0021889802022112 |
| [15] |
(a) L. Yang, X. Cui, Y. Zhang, Q. Yang, H. Xing, J. Mater. Chem. A 6 (2018) 24452-24458; (b) D.T. Vodak, M.E. Braun, J. Kim, M. Eddaoudi, O.M. Yaghi, Chem. Commun. (2001) 2534-2535; (c) D. Sun, R. Cao, Y. Sun, et al., Chem. Commun. (2003) 1528-1529. |
 2019, Vol. 30
2019, Vol. 30 

