The continuous rising morbidity and mortality of cancer have given rise to severe damage to the public health and economic development [1-4]. Developments of applicable strategies that can eliminate cancer become highly desired. Photodynamic therapy (PDT) is an attractive cancer treatment technique because it possesses many advantages, such as non-invasiveness, easyoperation, short treatment cycle and low side effects [5-8]. PDT involves three main components including photosensitizer, light and tissue oxygen. The excited photosensitizers can produce highly cytotoxic reactive oxygen species (ROS) to damage the tumor cells and induce their apoptosis [9-11]. However, the poor solubility and weak internalization of the conventional molecule-photosensitizers extremely impeded its therapeutic efficiency [12]. To overcome these shortcomings, many nanocarriers were prepared as the powerful delivery systems of molecule-photosensitizers, including inorganic nanoparticles, organic nanoparticles and liposomes [13-20]. But some new problems emerged, such as complex preparation process and compromised singlet oxygen generation capacity. Moreover, most of the nanocarriers are poorly biocompatible, which unavoidably leads to potential system toxicities in vivo. In addition, most of the photosensitizers locate in cytoplasm after enter cancer cells and the produced ROS displays exceedingly short lifespans and limited diffusion distance, which makes them incapable of causing serious cell damage and thus inevitably resulting in the low therapeutic efficiency of cancer as well [21-26]. Hence, it is necessary to develop a more facile and effective strategy to improve the therapeutic effect of PDT for tumor tissues.
Mitochondrion is one of the most significant organelles in living cells, which undertakes multiple important functions including regulating metabolic processes and producing most of cellular ROS [27-30]. Generally, cancer cells keep a modest amount of ROS to provide the requirements of cell proliferation. However, abnormal generation of ROS within mitochondria will lead to mitochondrial dysfunction, which is closely associated with cell apoptosis [31-33]. Thus, a large amount of photosensitizer accumulation in the mitochondria will result in continuous generation of ROS, which can be helpful for causing mitochondrial damage and further promoting cancer cell apoptosis rapidly [34, 35]. Therefore, the design and preparation of effective mitochondria-targeted photosensitizer is promising to improve the therapeutic efficiency of PDT.
Among available nanocarriers, mesoporous silica nanoparticles (MSN) are considered to be outstanding candidates for drug delivery on account of their excellent structure and properties, such as easy preparation, good thermal and chemical stabilities, tunable particle size, large specific surface area, easy surface modification and great biocompatibility [36, 37]. Moreover, MSN have been approved for clinical conversion by the Food and Drug Administration because of its good safety. Therefore, MSN have been used to delivery various cargoes into cancer cells for cancer therapy. However, the drug molecules were usually directly loaded into the pores of MSN, which would inevitably lead to their unexpected leakage. Therefore, it is very necessary to develop stable MSN-based drug loading system for improving cancer treatment.
Herein, we developed a mitochondria-targeted nanophotosensitizer for boosting the PDT efficiency. The small sized MSN was selected as the nanocarrier. After functionalized with amino groups of MSN, the molecular photosensitizer chlorin e6 (Ce6) was linked on the internal and external surfaces of the MSN by the formation of amide bonds. Then, the as-synthesize nanoparticles were covalently modified with triphenylphosphine (TPP), which is a kind of delocalized lipophilic cations that enables them to easily accumulate into highly negatively charged mitochondria [38]. The nanophotosensitizer (MSN-Ce6-TPP) could produce ROS in mitochondria upon laser irradiation, which would result in the mitochondrial dysfunctions and further cause the decrease of △Ψm and the increase of oxidation stress. The oxidation stress can, in return, induce more ROS production. The continuously decreased △Ψm would activate the caspase-3 and eventually cause irreversible cell death. Consequently, the enhanced treatment efficiency both in vitro and in vivo was successfully realized. The construction of the nanophotosensitizer and details of this strategy are displayed in Scheme 1.

|
Download:
|
| Scheme 1. Schematic illustration of the procedure for preparing MSN, MSN-Ce6, MSN-Ce6-TPP (a) and the laser-activated enhanced of photodynamic therapy efficiency of MSN-Ce6-TPP in vivo (b). | |
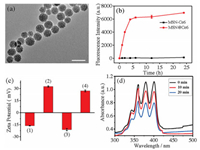
The small-sized aminated MSN (MSN-NH2) was prepared following the previously literature protocol. The average sizes of the MSN-NH2 and the nanophotosensitizer were about 30 nm (Fig. 1a and Fig. S1 in Supporting information). Ninhydrine experiments confirmed the successful functionalization of MSN with-NH2 groups (Fig. S2 in Supporting information). The mainly working mechanism of PDT is that the excited photosensitizer can transfer energy to the nearby triplet oxygen molecules, thus generating highly reactive singlet oxygen (1O2) to cause oxidation stress in cancer cells. Avoiding the fluorescence quenching is a critical protocol for ensuring the PDT efficiency. Therefore, we first optimized the concentration of Ce6 via fluorescent spectrum (Fig. S3 in Supporting information). From the obtained spectrum we found that the fluorescence intensities of the obtained MSNCe6 raised with the concentrations of Ce6 enhancement in the low concentration range; but decreased when high concentration of Ce6 was added into the reaction system, which can be attributed to the self-quenching phenomenon of the Ce6 molecules. Therefore, 15.0 mmol/L was selected as the optimal concentration of Ce6. In addition, the stability of MSN-Ce6 was studied by the timedependent experiments (Fig. 1b). It displayed that the Ce6 molecules loaded in MSN-NH2 (MSN@Ce6) by physical adsorption leaked obviously within 5 h, while no obvious leakage of Ce6 was observed from the MSN-Ce6 on the basis of covalent bonds linkage. It strongly demonstrated that the MSN-Ce6 had better stability than MSN@Ce6.
|
Download:
|
| Fig. 1. (a) HRTEM image of the MSN. Scale bar: 50 nm. (b) The leakage of Ce6 from MSN@Ce6 and MSN-Ce6 nanoparticles. (c) Zeta potential of the every step modification: (1) MSN, (2) MSN-NH2, (3) MSN-Ce6, (4) MSN-Ce6-TPP. (d) The absorption spectra of ABMD after treatment with MSN-Ce6-TPP and laser irradiation at different times. | |
Next, to endow the nanophotosensitizer with mitochondriatargeting capability, positively charged lipophilic delocalized group TPP was further modified onto the MSN-Ce6. The UV-vis absorption of the MSN-Ce6-TPP was further studied. The obvious absorption of MSN-Ce6 at 400 nm and 650 nm was observed (Fig. S4 in Supporting information), which was associated with the characteristic absorption of Ce6. Moreover, the maximum fluorescence intensity of the MSN-Ce6-TPP locates at 650 nm (Fig. S5 in Supporting information), suggesting that the emission characteristic of Ce6 have not been influenced after TPP modification. Subsequently, the zeta potential experiments were carried out. As shown in Fig. 1c, the different values of zeta potential confirmed the successful modification processes, i.e., -16.3 ± 0.3 mV (MSN), 32.6 ± 0.8 mV (MSN-NH2), -21.2 ± 1.4 mV (MSN-Ce6), 27.1 ±1.2 mV (MSN-Ce6-TPP). Based on the standard linear calibration curve (Fig. S6 in Supporting information), the content of Ce6 in MSN-Ce6 was determined to be 0.076 μmol/mg and 0.013 μmol/mg, respectively.
To further evaluate the 1O2 generating ability of nanophotosensitizer, the 9, 10-anthracenediyl-bis(methylene) dimalonic acid (ABMD) was used as an indicator [39]. The decreased absorbance of ABMD will be observed after ABMD reacted with 1O2. After the nanophotosensitizer was mixed with ABMD, the mixture solution was exposed to laser irradiation. Fig. 1d displayed that the absorbance intensity of ABMD decreased obviously with the increasing of irradiation time from 0 to 20 min, confirming that the MSN-Ce6-TPP could efficiently produce 1O2 under laser irradiation.
Then, the colocalization imaging experiments were performed to evaluate the mitochondria-targeting capability of MSN-Ce6-TPP. A commercial available mitochondrion-staining dye, mito-tracker green (MTG), was choose to co-stain mitochondria in 4T1 cells with the nanophotosensitizer. As show in Fig. S7 (Supporting information), a yellow fluorescence signal was clearly observed in the merged image, which could be attributed to the well matched of the red fluorescence from MSN-Ce6-TPP and the green fluorescence from MTG. The Pearson's correlation coefficient (PCC) ranges from -1 to 1, with -1, 0, 1 demonstrating a negative correlation, no correlation and positive correlation respectively [40, 41]. The PCC value was calculated to be 0.73 for MSN-Ce6-TPP. Moreover, the fluorescence intensity of the line scanning profiles was consistent with the above results. The control experiment was also performed using MSN-Ce6 nanoparticles without of the mitochondrial targeting group TPP (Fig. S8 in Supporting information). The PCC value was 0.33, revealing that the MSN-Ce6 nanoparticles exhibited inferior mitochondria targeting ability. All these results confirmed that the nanophotosensitizer displayed outstanding mitochondrion-targeting ability, which was promising to photoinduced mitochondrial ROS generation.
To assess the ROS generation ability of MSN-Ce6-TPP, a commercial probe 2', 7'-dichlorodihydrofluorescein diacetate (DCFH-DA) was used to image the intracellular ROS [42, 43]. As shown in Fig. S9 (Supporting information), the weak fluorescence signals were observed in the PBS or PBS with laser irradiation groups. Meanwhile, either the MSN-Ce6 or MSN-Ce6-TPP group without light exposure also displayed week fluorescence signals, implying a low production of ROS in the living cells. Upon laser irradiation, the cells treated with MSN-Ce6 or MSN-Ce6-TPP showed clearly bright green fluorescence, especially the cells treated with MSN-Ce6-TPP showed strongest fluorescence intensity. These results verified that the nanophotosensitizer can efficiently induce more ROS generation and cause mitochondrial dysfunction in cancer cells after light irradiation [44].
It has been reported that the decrease of △Ψm occurs in mitochondria trigger apoptosis process, because the over expressed ROS leads to the mitochondrial permeability transition pore opening by oxidation of glutathione [45]. We use rhodamine 123 (Rh123) as an indicator for the △Ψm [46]. As shown in Fig. S10 (Supporting information), without laser irradiation, the fluorescence intensity of the 4T1 cells incubated with either the MSN-Ce6 or MSN-Ce6-TPP did not change clearly compared with the PBS with or without laser irradiation groups, indicating the good biocompatibilities of the MSN based photosensitizers. After laser irradiation, the fluorescence intensity of Rh123 decreased in these two groups, confirming that the produced ROS could cause mitochondrial dysfunction of cancer cells. Obviously, the fluorescence intensity of the cells treated with MSN-Ce6-TPP was much lower than that of MSN-Ce6 group. It demonstrated that MSN-Ce6- TPP with laser irradiation could most efficiently weaken the △Ψm.
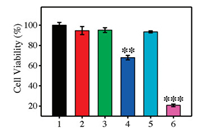
The increased production of ROS in 4T1 cells by MSN-Ce6-TPP maycause enhanced PDTefficacy to kill the cancer cells. Therefore, MTTassay was carried out to evaluate the potential applications of the nanophotosensitizers. As shown in Fig. 2, the group treated with laser irradiation remained high viability, indicating that the short time of light irradiation caused little damage to the cells. Meanwhile, the cells remained more than 90% viability after treated with MSN-Ce6 and MSN-Ce6-TPP, indicating that the nanoparticles were nontoxic to cells. However, the survival rate of the cells clearly declined after the cells treated with MSN-Ce6 or MSN-Ce6-TPP exposed to 655 nm laser. It was worth noting that mitochondria-targeted MSN-Ce6-TPP group displayed much higher cytotoxicity than non-targeted MSN-Ce6 group, which highlighted the strategy advantage of mitochondria targeting PDT. All these evidences clearly demonstrated that the mitochondria location could effectively boost the PDT efficiency of nanophotosensitizer.
|
Download:
|
| Fig. 2. Cell viability of 4T1 cells treated with different conditions. 1: PBS, 2: PBS+Laser, 3: MSN-Ce6, 4: MSN-Ce6 + Laser, 5: MSN-Ce6-TPP, 6: MSN-Ce6- TPP+Laser. Compared with the PBS-treated group, **P < 0.01; ***P < 0.001. | |
Caspase-3 activation is a significant signal in ROS-mediated apoptotic pathways [47]. Immunofluorescence staining method was used to investigate the caspase-3 activation during different treatments. Without laser irradiation, the fluorescence intensities of MSN-Ce6 or MSN-Ce6-TPP groups were comparable with the PBS groups, revealing weak activity of caspase 3 (Fig. S11 in Supporting information), whereas increased activity of caspase-3 was detected after the cells incubated with MSN-Ce6 or MSN-Ce6- TPP with laser irradiation. Noticeably, the increased caspase-3 activity of MSN-Ce6-TPP-treatedgroupwas much greaterthan that of MSN-Ce6 after laser irradiation, validating that the mitochondria-targeted nanophotosensitizer could more effectively activate ROS production and ultimately induce the cell apoptosis.
Inspired by the outstanding therapeutic effect in vitro, we next intend to assess the therapeutic efficacy of the mitochondria targeted nanophotosensitizer in vivo by calculating the tumor growth rate. As shown in Fig. 3b, the tumor size of the groups treated with PBS buffer, PBS with light irradiation, MSN-Ce6 and MSN-Ce6-TPP without light irradiation increased approximately 5.6-fold, implying that the MSN-Ce6 and MSN-Ce6-TPP have no therapeutic effect without light irradiation. The tumor growth in MSN-Ce6 with laser irradiation treatmentgroup was suppressedin the early days, but recurrence occurred later and resulted in 3.2- fold tumor volume increment. In contrast, the tumor growth of the MSN-Ce6-TPP-treated group with laser irradiation was found to completely suppressed, and much more pyknotic cells with highly condensed nuclei were observed from hematoxylin and eosin analysis (Fig. S12 in Supporting information), implying that the mitochondria -targeted nanophotosensitizers (MSN-Ce6-TPP) possessed higher therapeutic effects. Additionally, neither obvious body weight lose (Fig. 3c) nor pathological abnormalities of the main organs (Fig. S13 in Supporting information) were observed, revealing that these treatments had no obvious side eff; ects. Overall, these results demonstrated that the developed nanophotosensitizer had great potentials for highly efficient PDT of tumors.

|
Download:
|
| Fig. 3. (a) Photographs of the tumor bearing mice with different treatments: PBS only, PBS with laser irradiation, MSN-Ce6, MSN-Ce6 with laser irradiation, MSN-Ce6-TPP, MSN-Ce6-TPP with laser irradiation. (b) Tumor growth curves of mice in different groups. (c) The body weights curves of mice in different groups. For irradiation groups, tumor region were exposed to 655 nm laser at 0.1 W/cm2 for 10 min after intratumor injected 12 h. Compared with the PBS-treated group, **P < 0.01; ***P < 0.001. | |
In summary, we have developed a mitochondria-targeted nanophotosensitizer for enhanced PDT against cancer. Ce6 was covalently attached to the internal and external surfaces of the MSN with an optimized concentration for avoiding the fluorescence quenching and reducing the risks of drug leakage. Further modification of TPP endowed the nanoparticles selectively localize in mitochondria. Compared with the Ce6 directly loaded in MSN, the covalently linked nanophotosensitizer shows much higher stability, which can more effectively carry Ce6 into mitochondria. The confocal fluorescence imaging results demonstrated that high dosage of ROS was successfully generated after laser irradiation, which resulted in the continuously decrease of △Ψm and eventually causing the irreversible cell death. Therefore, the significant enhanced PDT efficiency was achieved both in vitro and in vivo. We anticipate that this simple and robust strategy inspires to develop more highly efficient organelle-targeted photosensitizers for clinical cancer treatment.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21535004, 91753111, 21874086, 21775094, 21505087, 21390411) and the Key Research and Development Program of Shandong Province (No. 2018YFJH0502).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2019.03.032.
| [1] |
J. Bai, X. Jia, W. Zhen, W. Cheng, X. Jiang, J. Am. Chem. Soc. 140 (2017) 106-109. |
| [2] |
P. Wang, L. Zhang, W. Zheng, et al., Angew. Chem. Int. Ed. 57 (2018) 1491-1496. DOI:10.1002/anie.v57.6 |
| [3] |
L. Cheng, C. Wang, L. Feng, K. Yang, Z. Liu, Chem. Rev. 114 (2014) 10869-10939. DOI:10.1021/cr400532z |
| [4] |
L. Feng, S. Gai, F. He, et al., J. Lin, Biomaterials 147 (2017) 39-52. DOI:10.1016/j.biomaterials.2017.09.011 |
| [5] |
K.L. Zhang, Y.J. Wang, J. Sun, et al., Chem. Sci. 10 (2019) 1555-1561. DOI:10.1039/C8SC03224F |
| [6] |
X. Zhang, F. Ai, T. Sun, et al., Inorg. Chem. 55 (2016) 3872-3880. DOI:10.1021/acs.inorgchem.6b00020 |
| [7] |
T.T. Xu, J.H. Li, F.R. Cheng, et al., Chin. Chem. Lett. 28 (2017) 1885-1888. DOI:10.1016/j.cclet.2017.07.029 |
| [8] |
D.E. Dolmans, D. Fukumura, R.K. Jain, Nat. Rev. Cancer 3 (2003) 380-387. DOI:10.1038/nrc1071 |
| [9] |
N. Li, H. Yang, W. Pan, W. Diao, B. Tang, Chem. Commun. 50 (2014) 7473-7476. DOI:10.1039/C4CC01009D |
| [10] |
A. Kamkaew, L. Cheng, S. Goel, et al., ACS Appl. Mater. Interfaces 8 (2016) 26630-26637. DOI:10.1021/acsami.6b10255 |
| [11] |
H. Fan, G. Yan, Z. Zhao, et al., Angew. Chem. Int. Ed. 55 (2016) 5477-5482. DOI:10.1002/anie.201510748 |
| [12] |
Z. Zhao, S. Shi, Y. Huang, S. Tang, X. Chen, ACS Appl. Mater. Interfaces 6 (2014) 8878-8885. DOI:10.1021/am501608c |
| [13] |
B. Poinard, S.Z.Y. Neo, E.L.L. Yeo, et al., ACS Appl. Mater. Interfaces 10 (2018) 21125-21336. DOI:10.1021/acsami.8b04799 |
| [14] |
I. Roy, T.Y. Ohulchanskyy, H.E. Pudavar, et al., J. Am. Chem. Soc. 125 (2003) 7860-7865. DOI:10.1021/ja0343095 |
| [15] |
H. Zhu, J. Li, X. Qi, P. Chen, K. Pu, Nano Lett. 18 (2017) 586-594. |
| [16] |
H. Wang, Y. Chao, J. Liu, et al., Biomaterials 181 (2018) 310-317. DOI:10.1016/j.biomaterials.2018.08.011 |
| [17] |
Y. Ma, X. Li, A. Li, et al., Angew. Chem. Int. Ed. 56 (2017) 13752-13756. DOI:10.1002/anie.201708005 |
| [18] |
T. Guo, Y. Wu, Y. Lin, et al., Small 14 (2018) 1702815. DOI:10.1002/smll.v14.4 |
| [19] |
C. Zhang, W.H. Chen, L.H. Liu, et al., Adv. Funct. Mater. 27 (2017) 1700626. DOI:10.1002/adfm.v27.43 |
| [20] |
C. Yao, W. Wang, P. Wang, et al., Adv. Mater. 30 (2018) 1704833. DOI:10.1002/adma.201704833 |
| [21] |
Q. Chen, Y. Ma, J. Zhao, et al., Chin. Chem. Lett. 29 (2018) 1171-1178. DOI:10.1016/j.cclet.2018.04.025 |
| [22] |
D. Zhang, L. Wen, R. Huang, et al., Biomaterials 153 (2018) 14-26. DOI:10.1016/j.biomaterials.2017.10.034 |
| [23] |
P. Rajaputra, G. Nkepang, R. Watley, Y. You, Bioorg. Med. Chem. 21 (2013) 379-387. DOI:10.1016/j.bmc.2012.11.032 |
| [24] |
C.J. Zhang, Q. Hu, G. Feng, et al., Chem. Sci. 6 (2015) 4580-4586. DOI:10.1039/C5SC00826C |
| [25] |
Y. Zheng, H. Lu, Z. Jiang, et al., J. Mater. Chem. B 5 (2017) 6277-6281. |
| [26] |
K. Liu, X. Liu, Q. Zeng, et al., ACS Nano 6 (2012) 4054-4062. DOI:10.1021/nn300436b |
| [27] |
H.W. Liu, X.X. Hu, K. Li, et al., Chem. Sci. 8 (2017) 7689-7695. DOI:10.1039/C7SC03454G |
| [28] |
T. Shpilka, C.M. Haynes, Nat. Rev. Mol. Cell Biol. 19 (2018) 109-120. DOI:10.1038/nrm.2017.110 |
| [29] |
L. Yang, N. Li, W. Pan, Z. Yu, B. Tang, Anal. Chem. 87 (2015) 3678-3684. DOI:10.1021/ac503975x |
| [30] |
X. Fang, Y. Wang, X. Ma, et al., J. Mater. Chem. B 5 (2017) 4190-4197. |
| [31] |
M.C. Wei, W.X. Zong, E.H.Y. Cheng, et al., Science 292 (2001) 727-730. DOI:10.1126/science.1059108 |
| [32] |
H. Lu, Y. Zheng, X. Zhao, et al., Angew. Chem. Int. Ed. 55 (2016) 155-159. DOI:10.1002/anie.201507031 |
| [33] |
J. Xu, P. Yang, M. Sun, et al., ACS Nano 11 (2017) 4133-4144. DOI:10.1021/acsnano.7b00944 |
| [34] |
N. Li, L. Yu, J. Wang, et al., Chem. Sci. 9 (2018) 3159-3164. DOI:10.1039/C7SC04458E |
| [35] |
Z. Yu, Q. Sun, W. Pan, N. Li, B. Tang, ACS Nano 9 (2015) 11064-11074. DOI:10.1021/acsnano.5b04501 |
| [36] |
S. Shi, F. Chen, S. Goel, et al., Nano-Micro Lett. 10 (2018) 65-68. DOI:10.1007/s40820-018-0216-2 |
| [37] |
P.N. Durfee, Y.S. Lin, D.R. Dunphy, et al., ACS Nano 10 (2016) 8325-8345. DOI:10.1021/acsnano.6b02819 |
| [38] |
X.W. Hua, Y.W. Bao, Z. Chen, F.G. Wu, Nanoscale 9 (2017) 10948-10960. DOI:10.1039/C7NR03658B |
| [39] |
X.F. Qiao, J.C. Zhou, J.W. Xiao, et al., Nanoscale 4 (2012) 4611-4623. DOI:10.1039/c2nr30938f |
| [40] |
W. Chen, W. Deng, E.M. Goldys, Mol. Ther. Nucl. Acids 7 (2017) 366-377. DOI:10.1016/j.omtn.2017.04.015 |
| [41] |
W. Chen, W. Deng, X. Xu, et al., J. Mater. Chem. B 6 (2018) 5269-5281. DOI:10.1039/C8TB00994E |
| [42] |
W. Chen, J. Ouyang, H. Liu, et al., Adv. Mater. 29 (2018) 1603864. |
| [43] |
H. Cheng, R.R. Zheng, G.L. Fan, et al., Biomaterials 188 (2019) 1-11. DOI:10.1016/j.biomaterials.2018.10.005 |
| [44] |
M.A. Aon, S. Cortassa, B. O'Rourke, Natl. Acad. Sci. U. S. A. 101 (2004) 4447-4452. DOI:10.1073/pnas.0307156101 |
| [45] |
J.D. Ly, D.R. Grubb, A. Lawen, Apoptosis 8 (2003) 115-128. DOI:10.1023/A:1022945107762 |
| [46] |
H. Zhang, Z. Yi, Z. Sun, et al., J. Mater. Chem. B 5 (2017) 7622-7631. DOI:10.1039/C7TB01323J |
| [47] |
Z. Xu, D. Kong, X. He, et al., Inorg. Chem. Front. 5 (2018) 2100-2105. DOI:10.1039/C8QI00476E |
 2019, Vol. 30
2019, Vol. 30 

