b Shanghai Electrochemical Energy Devices Research Center, School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China
Non-aqueous lithium-oxygen (Li-O2) batteries have recently aroused extensive interest as promising energy storage systems with ultrahigh energy density and are expected to be useful in electric vehicles (EVs) and smart power grids [1-8]. Now electrolytes used in the Li-O2 batteries are mainly organic liquid electrolytes normally prepared by dissolving lithium salts into organic solvents. After the liquid electrolyte screening in the past ten years, tetra(ethylene) glycol dimethyl ether (TEGDME), one of ethers organic solvents, is now widely used electrolytes in the Li-O2 batteries due to its relative chemical stability toward oxygen and low volatility [9]. However, further investigations indicate that TEGDME-based electrolyte upon cycling in the Li-O2 batteries will undergo attack by the superoxide radical and decompose to form Li2CO3, HCO2Li, CH3CO2Li, and polyethers/ ethers, which lead to capacity rapid fading [10]. In addition, there are still some safety concerns limited the TEGDME based liquid electrolytes used in Li-air batteries since due to their flammable and volatility [11, 12].
Ionic liquids such as 1-methyl-1-butyl pyrrolidinium bis (trifluoromethanesulfonyl)imide and 1-ethyl-3-methyl imidazolium bis(trifluoromethylsulfonyl)amide exhibit promising stability toward the superoxide radicals [13, 14]. However, ionic liquids still possess several shortcomings: low Li+ transfer number, low lithium salt solubility, low ionic conductivity and moisture susceptibility, which prevent their practical use in the Li-O2 batteries [15].
Organic ionic plastic crystals (OIPCs) have been pursued as next-generation solid-state electrolytes due to their good thermal and electrochemical stability, non-flammability and non-volatility [16, 17]. Since their liquid-like behavior within a crystalline lattice, it allows high diffusivity of Li+ ions through rotational and translational motions of the matrix ions. Pure OIPCs show low ionic conductivity at room temperature, however, doping lithium salts such as lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) and lithium bis(fluorosulfonyl)imide (LiFSI) will remarkably increase ionic conductivity over 10-3 S/cm at room temperature [18]. Due to these unique properties, the OIPCs have attracted more attentions used as electrolytes for the application in electrochemical energy devices such as lithium ion batteries [19, 20], sodium ion batteries [21], fuel cells [22] and dye-sensitized solar cells [23, 24]. To the best of our knowledge, however, there are not any reports about the OIPCs used as electrolytes for the Li-O2 batteries.
Herein, we reported 1-ethyl-1-methyl pyrrolidinium bis(fluorosulfonyl)imide (P12FSI) doping LiFSI salt used as electrolyte for the Li-O2 batteries. P12FSI is a well-known organic ionic plastic crystal, with an endothermic solid-solid phase transition at -22 ℃ and a melting point at about 203 ℃ [19].Pyrrolidinium-based ionicliquids such as containing 1-methyl-1-propyl pyrrolidium bis(trifluoromethanesulfonyl)imide(P13TFSI) and1-methyl-1-butyl pyrrolidium bis (trifluoromethanesulfonyl)imide(PP14TFSI)are considered as suitable electrolytes for the Li-O2 batteries due to their good electrochemical and thermal stability [25]. Due to the chemical structure of P12FSI is similar to the chemical structures of P13TFSI and PP14TFSI, therefore weexpectthattheP12FSIOIPC will be used as electrolyte for the Li-O2 batteries. Inourexperiments, P12FSI and LiFSI mixture (mole ratio of 1:1) was used as the electrolyte, and evaluated its performance including chemical stability against the superoxide radicals, electrochemical stability, ionic conductivity, cycling stability and rate capability of the Li-O2 batteries.
P12FSI was first synthesized with 1-methylpyrrolidine and bromoethane and then obtained by an anion exchange with LiFSI salt, which is described in the previous report [26]. To prepare the P12FSI-LiFSI electrolyte, P12FSI and LiFSI were mixed with the mole ratio of 1:1 and stirred for 24 h at room temperature under argon atmosphere. The chemical structure of P12FSI was analyzed by nuclear magnetic resonance (NMR, MERCURY plus 400, Varian), using DMSO-d6 as solvent and tested at room temperature. Thermal behaviors of the sample were analyzed by differential scanning calorimeter (DSC, Q2000, TA Instruments). DSC testing temperature was from -100 ℃ to 230 ℃ at the rate of 10 ℃/min. Linear sweep voltammetry (LSV, CHI660E, CH Instruments Ins.) was carried out at a scanning rate of 1mV/s at room temperature. The tested cell was constructed by sandwiching glass fiber filled with electrolyte between stainless steel and lithium foil. Ionic conductivity was measured by AC impedance method using Autolab (PGSTAT302, Eco Chemie, Netherlands) with the frequency ranging from 100 kHz to 0.1 Hz and a potential amplitude of 50mV.
Potassium superoxide (KO2) screening method was employed to evaluate the chemical stability of the P12FSI-LiFSI electrolyte in the presence of superoxide radical. An amount of KO2 powder was added in 2 mL of the P12FSI-LiFSI electrolyte and stirred throughout the experiments at room temperature. Next, 0.3 mL of themixedsolution at different time point was transferred to an NMR tube containing DMSO-d6 solvent and tested for 1H NMR analysis. All experiments (except 1H NMR) were performed in an argon-filled glove box.
The cathodes were composed of multi-wall carbon nanotubes (Shenzhen Nanotech Port Co., Ltd., China) and poly(vinylidene fluoride) (PVDF). The weight ratio of MWCNTs:PVDF was 8:2. The loading of MWCNTs in a cathode was about 1.0 mg/cm2. A Li-O2 battery was comprised of a modified 2032 coin-cell with six holes, a lithium metal anode (1.56 cm diameter and 0.45 mm thick), a Whatman glass filter separator (~20 mm diameer) impregnated with electrolyte and a prepared cathode. After assembled in an argon-filled glove box, the batteries were placed in a gastight box with high-purity oxygen (99.999%) atmosphere and tested on Land CT2001A test system (Wuhan, China).
Fig. 1a showed the 1H NMR spectrums of pure P12FSI. The spectrum was in good agreement with the expected structure. There were six peaks at 1.28 ppm, 2.09 ppm, 2.50 ppm (solvent DMSO-d6 peak), 2.96 ppm, 3.36 ppm and 3.44 ppm, the corresponding H atoms were marked by numbers, respectively. The integrated area ratio (from 1 to 5) of the five peaks was 4.00:4.13:3.05:2.05:3.05, which was well matched with the number of H atoms (4:4:3:2:3). The chemical stability of the P12FSI-LiFSI electrolyte was studied by the potassium superoxide (KO2) screening method. To simulate the environment of Li-O2 battery, KO2 powders were added to the P12FSI-LiFSI electrolyte to generate O2- species. We found that the P12FSI-LiFSI electrolyte containing KO2 powders showed no colour change after 3 days (Fig. 1b). And there was no color change even after 30 days for the P12FSI-LiFSI electrolyte containing KO2 powders. Compared to the 1H NMR spectrum of pristine P12FSI as shown in Fig. 1a, there was notanychange of the P12FSI-LiFSIelectrolyte containing KO2 after 3 days. These results indicated that the P12FSI-LiFSI electrolyte showed good chemical stability against superoxide radicals.

|
Download:
|
| Fig. 1. (a) 1H NMR spectrum of P12FSI (pristine) and the P12FSI-LiFSI electrolyte after adding KO2 powders for 3 days. (b) Photographic images of (Ⅰ) P12FSI-LiFSI electrolyte (Ⅱ) after adding KO2 powders for 10s and (Ⅲ) after 72 h. (c) DSC curves of the P12FSI and the P12FSI-LiFSI electrolyte. | |
Differential scanning calorimetry (DSC) curve (Fig. 1c) showed that the P12FSI underwent solid-solid transitions at -72 ℃ and -22 ℃ and melted at 203 ℃, respectively. At room temperature, the pure P12FSI is a waxy solid. When the same mole of LiFSI salts was added into the P12FSI, these two solid materials were slowly transformed into liquid and finallybecame viscous and transparent colorless liquid (Fig. 1b). From the DSC curve of the P12FSI-LiFSI as shown in Fig. 1b, it can be found that the solid-solid transition disappeared, Tg appeared at about -81 ℃ and a newcrystallization peak was observed at around 125 ℃.
High ionic conductivity at room temperature of the electrolyte is an important factor, which will directly affect the electrochemical performance of Li-O2 batteries. The ionic conductivity of pure P12FSI was 1.23 × 10-7 S/cm at room temperature [19, 20], which is not suitable for the application of Li-O2 batteries. As reported previously, doping lithium salts will significantly improve the ionic conductivity of the plastic crystal system [18]. Therefore, 50 mol% of LiFSI was added to P12FSI in our experiments. It can be found that the ionic conductivity dramatically increased. At room temperature, the ionic conductivity of the P12FSI-LiFSIelectrolyte(2.20 × 10-3 S/cm) showed about four orders of magnitude higher than that of the pure P12FSI.As shown in Fig. 2a, the temperature dependence of the ionic conductivity of P12FSI-LiFSI electrolyte agrees with the Vogel-Tamman-Fulcher (VTF) Eq.(1) over the temperature range examined.
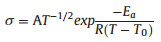
|
(1) |
A is pre-exponential factor, Ea is activation energy, R is gas constant and T0 is deal transition temperature at which the configurational entropy is zero.
Electrochemical stability of P12FSI-LiFSI electrolyte was evaluated by the linear sweep voltammetry (LSV) test. As shown in Fig. 2b, it can be observed that the electrolyte remained stable until the voltage went up to 5.3 V vs. Li/Li+. In addition, it can be found that P12FSI-LiFSI electrolyte showed non-flammability (Fig. 2c).
|
Download:
|
| Fig. 2. (a) Temperature dependence of ionic conductivity of the P12FSI-LiFSI electrolyte. (b) Linear sweep voltammetry of the P12FSI-LiFSI electrolyte with a scan rate 1mV/s under O2 atmosphere and room temperature. (c) Flammability test of the P12FSI-LiFSI electrolyte. | |
A Li-O2 battery was fabricated with the P12FSI-LiFSI electrolyte impregnated into a Whatman glass fiber separator, an air-electrode made of multi-wall carbon nanotubes (MWCNTs), PVDF binder without any catalysts and a lithium metal anode. Fig. 3a showed the initial discharge and charge curves of the Li-O2 battery under constant current density of 200 mA/g in voltage range of 2.0-4.5 V at room temperature. The initial discharge voltage was about 2.5 V and gradually decreased to 2.0 V as the discharge process continued. The discharge and charge capacity were about 865 mAh/gcarbon and 631 mAh/gcarbon (round-trip efficiency 72.9%), respectively. Fig. 3b showed the rate capability with a fixed discharge capacity of 500 mAh/gcarbon under different current density. It can be found that the batteries can operate at high current rate with limited charge-discharge polarization. Fig. 3c showed the cycling stability of the battery cycled at a fixed capacity of 500 mAh/gcarbon and a current density of 200 mAh/g. This battery can be operated stably for 320 cycles, the discharge voltage remained up to 2.5 V and charge voltage remained below 5.0 V. The Li-O2 battery using normal organic liquid electrolytes (1 mol/L LiTFSI in TEGDME) can only keep 120 cycles with a fixed discharge capacity of 500 mAh/gcarbon at room temperature. All these results indicate that Li-O2 batteries with P12FSI-LiFSI electrolyte showed excellent cycling stability and good rate capability at room temperature.

|
Download:
|
| Fig. 3. Electrochemical performance of Li-O2 batteries using the P12FSI-LiFSI electrolyte at room temperature. (a) Initial discharge-charge voltage profiles at a current density of 200 mA/g. (b) Rate capability of the batteries at different current densities and a fixed capacity of 500 mA h/gcarbon. Variation of voltage on the terminal of discharge and charge of the batteries cycled at a fixed capacity of 500 mAh/g and current density of 200 mA/g. (c) Using P12FSI-LiFSI electrolyte and (d) TEGDME-1 mol/L LiTFSI electrolyte. | |
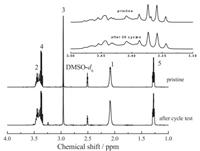
The electrochemical stability of the P12FSI-LiFSI electrolyte after battery cycling was investigated by 1H NMR. We test the electrolyte after running 200 cycles at a fixed capacity of 500 mAh/gcarbon and current density of 200 mA/g in the Li-O2 battery. As shown in Fig. 4, the peaks were the same to the pristine electrolyte, and there is no new peak compared to Fig. 1a. These results clearly demonstrated the electrochemical stability of the P12FSI-LiFSI electrolyte during the charge and discharge process of Li-O2 battery.
|
Download:
|
| Fig. 4. 1H NMR spectrum of P12FSI (pristine) and the P12FSI-LiFSI electrolyte after cycle test in Li-O2 batteries under a fixed capacity of 500 mAh/gcarbon and current density of 200 mA/g at room temperature. | |
In summary, we demonstrated that a Li-O2 battery can be operated by using organic ionic plastic crystal as electrolyte at room temperature. Since P12FSI-LiFSI electrolyte exhibited high ionic conductivity, good chemical stability and wide electrochemical window, the battery showed good rate capability, excellent cycling stability and can be operated stably for 320 cycles under a fixed capacity of 500 mAh/gcarbon. Considering a discharge voltage of 2.5 V and a reversible capacity limited to 500 mAh/gcarbon, the theoretical energy density of our battery may be calculated to be 1250 Wh/kg, which is much higher than that of the conventional lithium-ion battery cathodes, typically ranging from 600 mAh/g to 700 mAh/g. Our initial feasibility study presented here shows that the organic ionic plastic crystal electrolytes provide a promising strategy for the development of new electrolytes for Li-O2 batteries with high electrochemical performance and good safety.
AcknowledgmentsThis research was supported by the National Key R & D Program of China (No. 2016YFB0901505), National Natural Science Foundation of China (No. 21573145).
| [1] |
P. Bruce, S. Freunberger, L. Hardwick, J. Tarascon, Nat. Mater. 11 (2012) 19-29. DOI:10.1038/nmat3191 |
| [2] |
C. Wu, C. Liao, L. Li, J. Yang, Chin. Chem. Lett. 27 (2016) 1485-1489. DOI:10.1016/j.cclet.2016.03.023 |
| [3] |
T. Li, C. Wu, H. Yuan, L. Li, J. Yang, Chin. Chem. Lett. 28 (2017) 2155-2158. DOI:10.1016/j.cclet.2017.09.021 |
| [4] |
W. Chen, Y. Gong, J. Liu, Chin. Chem. Lett. 28 (2017) 709-718. DOI:10.1016/j.cclet.2016.10.023 |
| [5] |
R. Su, X. Zhang, J. Wang, et al., ACS Catal. 8 (2018) 4082-4090. DOI:10.1021/acscatal.7b04401 |
| [6] |
X. Zhang, Y. Gong, S. Li, C. Sun, ACS Catal. 7 (2017) 7737-7747. DOI:10.1021/acscatal.7b02153 |
| [7] |
C. Sun, F. Li, C. Ma, et al., J. Mater. Chem. A 2 (2014) 7188-7196. DOI:10.1039/C4TA00802B |
| [8] |
S. Liu, W. Zhang, N. Chen, C. Sun, ChemElectroChem 5 (2018) 2181-2185. DOI:10.1002/celc.201800426 |
| [9] |
H. Jung, J. Hassoun, J. Park, Y. Sun, B. Scrosati, Nat. Chem. 4 (2012) 579-585. DOI:10.1038/nchem.1376 |
| [10] |
Z. Peng, S. Freunberger, Y. Chen, P. Bruce, Science 337 (2012) 563-566. DOI:10.1126/science.1223985 |
| [11] |
J. Yi, S. Guo, P. He, H. Zhou, Energy Environ. Sci. 10 (2017) 860-884. DOI:10.1039/C6EE03499C |
| [12] |
Y. Liu, P. He, H. Zhou, Adv. Energy Mater 8 (2018) 17101602. |
| [13] |
M. Ara, T. Meng, G. Nazri, S. Salley, K. Ng, J. Electrochem. Soc. 161 (2014) A1969-A1975. DOI:10.1149/2.0031414jes |
| [14] |
G. Elia, J. Hassoun, W. Kwak, et al., Nano Lett. 14 (2014) 6572-6577. DOI:10.1021/nl5031985 |
| [15] |
T. Zhang, H. Zhou, Angew. Chem. Inter. Ed. 51 (2012) 11062-11067. |
| [16] |
J. Pringle, Phys. Chem. Chem. Phys. 15 (2013) 1339-1351. DOI:10.1039/C2CP43267F |
| [17] |
D. MacFarlane, M. Forsyth, Adv. Mater. 13 (2001) 957-966. DOI:10.1002/1521-4095(200107)13:12/13<957::AID-ADMA957>3.0.CO;2-# |
| [18] |
D. MacFarlane, J. Huang, M. Forsyth, Nature 402 (1999) 792-794. DOI:10.1038/45514 |
| [19] |
Y. Zhou, X. Wang, M. Forsyth, et al., Phys. Chem. Chem. Phys. 19 (2017) 2225-2234. DOI:10.1039/C6CP07415D |
| [20] |
Y. Zhou, X. Wang, M. Armand, et al., ChemSusChem 10 (2017) 3135-3145. DOI:10.1002/cssc.v10.15 |
| [21] |
F. Makhlooghiazad, D. Gunzelmann, M. Armand, et al., Adv. Energy Mater. 7 (2017) 1601272. DOI:10.1002/aenm.201601272 |
| [22] |
U. Rana, M. Forsyth, D. MacFarlane, J. Pringle, Electrochim. Acta 84 (2012) 213-222. DOI:10.1016/j.electacta.2012.03.058 |
| [23] |
D. Xu, C. Shi, L. Wang, L. Qiu, F. Yan, J. Mater. Chem. A 2 (2014) 9803-9811. DOI:10.1039/c4ta01255k |
| [24] |
S. Li, L. Qiu, C. Shi, X. Chen, F. Yan, Adv. Mater. 26 (2014) 1266-1271. DOI:10.1002/adma.v26.8 |
| [25] |
M. Piana, J. Wandt, S. Meini, et al., J. Electrochem. Soc. 161 (2014) A1992-A2001. DOI:10.1149/2.1131412jes |
| [26] |
M. Yoshizawa-Fujita, E. Kishi, M. Suematsu, T. Takekawa, M. Rikukawa, Chem. Lett. 43 (2014) 1909-1911. DOI:10.1246/cl.140833 |
 2019, Vol. 30
2019, Vol. 30 

