b Tianjin Key Laboratory of Advanced Fibers and Energy Storage, College of Materials Science and Engineering, Tianjin Polytechnic University, Tianjin 300387, China
Electric double layer capacitor (EDLC), also called supercapacitor, is a new type of energy storing device, which has longer service life, shorter charging time and higher power density than batteries [1-4]. As one of the most important parts of EDLCs, the electrolyte plays a decisive role in providing charged ions and as an ion transport medium. The physical and chemical properties of electrolyte determine the performance of the supercapacitor directly. Although the commercial electrolyte TEA-BF4 is widely used due to its low cost, its low solubility in solvents limits the operating temperature range of the supercapacitor. More importantly, the low operating voltage is the main reason for the low energy density of the supercapacitor. Therefore, it is urgent to develop organic electrolytes with high solubility and high chemical stability.
As the cyclic ammonium salt has better stability than the short chain fatty quaternary ammonium salt, more and more attention has been paid to the cyclic quaternary ammonium salts as electrolytes for EDLCs in the scientific community [5-9]. Among them the most studied quaternary ammonium salt is SBP-BF4 which has smaller volume and more stable chemical structure than traditional electrolyte. Our previous work has also demonstrated that spiro-(1, 1')-bipyrrolidinium tetrafluoroborate (SBP-BF4) is a new electrolyte with typical capacitance characteristics and excellent chemical stability on activated carbon supercapacitors. Investigations examining the performance of cations with varying structures suggest that ion size is the critical property that primarily contributes to increase ionic conductivity and pore diffusion [10-11]. The increase in pore accessibility allows more cations to populate the double layer of the activated carbon electrode resulting in higher capacitance [12-15]. Therefore, in theory, small ions size can easily enter the micropores of activated carbon electrode and occupy more surface area, furthermore, the distance between the center of the ion charge and the surface of the electrode will also be shortened.
Due to the electrochemical window of cyclic pyrrolidine ammonium salt are similar with short chain fatty quaternary ammonium salt, and have higher solubility in the solvent PC [16-17]. In this work, the smallest pyrrolidine quaternary ammonium salt N, N-dimethylpyrrolidinium tetrafluoroborate (P11-BF4) was synthesized first. Detailed ion size information is shown in Fig. S1 (Supporting information). The conductivity and the electrochemical stability windows of P11-BF4/PC were compared with conventional electrolyte TEA-BF4/PC. Finally, the AC impedance, cyclic voltammograms, galvanostatic charge-discharge and rate capacity of the EDLCs were investigated to obtain the best performance parameters.
All the reagents (N-methylpyrrolidine, dimethylcarbonate, fluoroboric acid, methanol, isopropanol, acetonitrile) used in the experiment were purchased from Mclean and Aladdin. TEA-BF4/PC electrolyte and PC solvent were got from Shenzhen CAPCHEM Technology Co., Ltd. EDLCs electrodes were prepared by mixing activated carbon (AC), carbon black (CB), polytetrafluoroethylene (PTFE). The type of activated carbon used is commercial (YP-50 F). The homogeneous slurry composition of AC:CB:PTFE was 82:10:8 wt%. The slurry will be mixed evenly with glass rods, then apply the rolling machine to the Al foil, after rolling the roller press out the electrode piece with diameter 13 mm with the thickness of about 100 μm. The standard button type EDLCs was fabricated using two activated carbon electrodes, a separator, a small amount of electrolyte. Finally, cover the positive shell and seal it with a sealing machine.
The total synthetic route of P11-BF4 is displayed in Fig. S2 (Supporting information).
N, N-Dimethylpyrrolidinium methylcarbonate [16]: N-Methylpyrrolidine (17.03 g, 0.25 mol) and dimethyl carbonate (112.6 g, 1.25 mol) was put in a 500 mL round bottomed flasks, then stirred for 48 h at 80 ℃. After the cooling of the reaction liquid, the excess of the unreacted raw materials are evaporated with a rotary evaporator at 70 ℃ to give a highly hygroscopic yellow solid. The main product is intermediates compound (P11-CH3OCO2).
N, N-Dimethylpyrrolidinium tetrafluoroborate: To 50 mL of a 1 mol aqueous solution of P11-CH3OCO2 was added dropwise about an equivalent of 50 wt% HBF4 until pH 5~6. The mixture reacted for an additional 12 h. The solvent and gaseous by-products were then removed on a rotary evaporator. The salts were first recrystallized three times with methanol/isopropanol, then dissolve the product in acetonitrile and adsorbed by activated carbon, filtered with neutral alumina and dried for 48 h at 80 ℃ in vacuum drying oven. The yield of target product P11-BF4 was 16.9 g, 91.0%. 1H NMR (500 MHz, D2O): δ 3.41~3.45 (4H, -CH2), 3.06 (6H, -N-CH3) and 2.13~2.18 (4H, -CH2-CH2) are approximately 2:3:2. 19F NMR: δ -150.41.
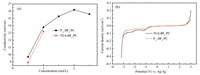
1H NMR and 19F NMR spectra were performed on a Bruker AVANCE 400 M spectrometer. The electrical conductivity of the electrolyte was measured using a conductivity meter (Mettler Toledo S30, Switzerland) in high and low temperature test box. The potential window was tested by an open three-electrode cell system with a glassy carbon electrode, Pt counter electrode and Ag/AgNO3 reference electrode at room temperature. CV curves for the assembled EDLCs were tested by electrochemical workstation (CHI604 A) at different voltages. The charge/discharge curves of the cells were tested by constant current method, and the charge discharge behavior and cycle performance of the cells were studied. Interfacial contact resistance and the series resistance was got by the AC impedance tested by the EIS plots (Autolab PGSTAT128 N, Switzerland), the frequency range from 104 Hz to 1 mHz. Fig. 1a shows the relationship between conductivity and concentration of P11-BF4/PC and TEA-BF4/PC. We can see that the maximum solubility of P11-BF4 in PC is 2.5 mol/L, while only 1 mol/L for TEA-BF4. For P11-BF4/PC, the conductivity increases with the increase of concentration at the initial stage, and decreases when the concentration is more than 2. The conductivity of P11-BF4 at each concentration is higher than that of TEA-BF4, which is the advantage brought by high solubility. High electrical conductivity lays a foundation for high power performance of supercapacitors.
|
Download:
|
| Fig. 1. (a) The conductivity of P11-BF4/PC and TEA-BF4/PC at different concentrations. (b) LSVs of 1 mol/L P11-BF4/PC and TEA-BF4/PC electrolyte systems measured on glassy carbon electrode, Pt counter electrode; Reference electrode: Ag/AgNO3; the scan rate is 5 mV/s. | |
Electrochemical stability of P11-BF4/PC electrolyte and TEA-BF4/PC electrolyte were tested by linear sweep voltammetry. Fig. 1b exhibits the electrochemical window of P11-BF4/PC and TEA-BF4/PC electrolytes. Both the two electrolytes exhibit wide electrochemical window and good electrochemical properties. It is worth noting that the peak appearing around 1 V vs. Ag/Ag+ may be related to the decomposition of trace moisture remaining in the electrolyte. The reduction potential is determined by the decomposition of BF4-, and the oxidation potential by TEA+ and P11+. The two electrochemical windows are close to 5.3 V. There is little difference between traditional electrolyte TEA-BF4/PC, so it can satisfy the requirement of EDLCs to the electrolyte.
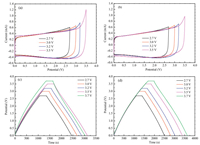
Figs. 2a and b show cyclic voltammograms of EDLCs assembled with P11-BF4/PC and TEA-BF4/PC electrolytes at different operating voltages. The cyclic voltammetry curve of the capacitors assembled with two electrolytes remained a good rectangle when the voltage range was increased from 0-2.7 V to 0-3.2 V. However, the rectangular variation of cyclic voltammetry curve of P11-BF4/PC electrolyte capacitor is smaller thanthatof capacitorassembled with TEA-BF4/PC electrolyte when the voltage range is increased to 0-3.5 V. We can get it from the graph that the P11-BF4/PC has higher working voltage than the TEA-BF4/PC, we can draw a conclusion that P11-BF4/PC electrolyte are more stable on the activated carbon. Therefore, the voltage range of P11-BF4/PC is slightly wider than that of TEA-BF4/PC electrolyte. Similar results can be obtained from GCD tests. Figs. 2c and d shows the galvanostatic charge-discharge profiles of P11-BF4 and TEA-BF4 in PC electrolytes based EDLCs under various charging voltages. According to the diagram, when the working voltage is below 3.5 V, the constant current chargedischarge curves of EDLCs assembled by P11-BF4 and TEA-BF4 both show isosceles trapezoid, the charge-discharge voltage varies linearly with time, and no redox capacitance occurs. When the working voltage is raised to 3.7 V, the charge discharge curve of TEA-BF4/PC begins to deform and is no longer isosceles trapezoid, while the shapeofP11-BF4/PCwaswell maintained.From the above results, it can be concluded that P11-BF4/PC has higher conductivity and voltage resistance, and is more suitable as a high voltage electrolyte.
|
Download:
|
| Fig. 2. Cyclic voltammetry curves of activated carbon electrode in different voltage regions of (a) P11-BF4/PC and (b) TEA-BF4/PC, the scan rate is 1 mV/s. Charge-discharge curves of the EDLCs with (c) P11-BF4/PC and (d) TEA-BF4/PC. The working voltage: 0~2.7 V to 0~3.7 V; The current density: 100 mA/g. | |
Fig. 3 shows the cyclic performance tests of the EDLCs assembled by the P11-BF4/PC electrolyte and TEA-BF4/PC electrolyte. When the voltage is 2.7 V, both electrolytes show excellent cyclic stability, but the gap between the two electrolytes gradually becomes prominent as the voltage increases. Especially when the voltage is 3.5 V, theTEABF4/PC attenuates seriously and cannot work normally. In contrast, the performance of P11-BF4/PC is obviously better than that of the TEA-BF4/PC. This result proves again that the voltage resistance of P11-BF4 is superior to that of TEA-BF4 electrolyte.

|
Download:
|
| Fig. 3. The cycle life of EDLCs with 1.0 mol/L P11-BF4/PC and TEA-BF4/PC carried out using a cell voltage of (a) 2.7 V (b) 3.0 V (c) 3.2 V (d) 3.5 V at a current density of 500 mA/g. | |
High power performance is one of the majoradvantages of EDLCs over batteries', it required that EDLCs must has good capacity retention at high discharge current. Fig. 4a shows the change of the specific capacitance of the EDLCs at different current densities. The test voltage is 2.7 V, and the current density range from 0.5A/g to 10 A/g. It can be seen from the diagram that the specific capacity of P11-BF4/PC electrolyte is obviously larger than that of TEA-BF4/PC electrolyte, especially at high current density, when the current density is increased from 0.5 A/g to 10 A/g, the capacitance retention rate of the EDLCs with P11-BF4/PC electrolyte system is about 55%, while that of the EDLCs with P11-BF4/PC electrolyte system is only about30%.This demonstrates the better rate performance of P11-BF4/PC electrolyte than that of TEA-BF4/PC electrolyte. The energy and power density displayed by the investigated EDLCs at different current densities were calculated and compared in a Ragone-likeplot (Fig. 4b). The cell performance of P11-BF4/PC is much better than TEA-BF4/PC, the maximum energy density and power density could reach to 29.73 Wh/kg and 13.75 kW/kg, while 27.74 Wh/kg and 12.04 kW/kg for TEA-BF4/PC, respectively.

|
Download:
|
| Fig. 4. (a) The charge/discharge rates performance of EDLCs with two electrolytes at current density range from 100 mA/g to 10 A/g. (b) Ragone plots for the EDLCs with two electrolytes. | |
In general, P11-BF4 was successfully synthesized and its conductivity and electrochemical window were characterized for the first time. New electrolyte salts are dissolved into the solvent PC for supercapacitor. Compared to commercial electrolyte, the specific capacity of P11-BF4/PC is higher than that of TEA-BF4/PC demonstrates that the smaller P11+ can easier enter the micropores of activated carbon and occupy more surface area, and the distance from the charge center of P11+ to the static charge on the electrode is smaller, cause an increase in discharge capacitance. More superior rate characteristics make P11-BF4 an ideal substitute for TEA-BF4 to realize high power supercapacitors.
AcknowledgementThis work was financially supported by the Guangdong Power Grid Co., Ltd. (No. GDKJXM20160000).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.01.007.
| [1] |
Y. Yan, Y.Q. Luo, J.Y. Ma, et al., Small 14 (2018) 1801815. DOI:10.1002/smll.v14.33 |
| [2] |
S.S. Zheng, H.G. Xue, H. Pang, Coord. Chem. Rev. 373 (2018) 2-21. DOI:10.1016/j.ccr.2017.07.002 |
| [3] |
Y. Yan, P. Gu, S.S. Zheng, et al., J. Mater. Chem. A 4 (2016) 19078-19085. DOI:10.1039/C6TA08331E |
| [4] |
J. Xu, Y. Wang, S. Cao, J. Mater. Chem. A 6 (2018) 17329-17336. DOI:10.1039/C8TA05976D |
| [5] |
Y. Nono, M. Kouzu, K. Takei, et al., Electrochemistry 78 (2010) 336-338. DOI:10.5796/electrochemistry.78.336 |
| [6] |
X.W. Yu, D.B. Ruan, C.C. Wu, et al., Power Sources (2014) 309-316. |
| [7] |
K. Chiba, T. Ueda, H. Yamamoto, Electrochemistry 75 (2012) 664-667. |
| [8] |
H.M. Zhou, W.J. Sun, J.J. Li, Cent. South Univ. 22 (2015) 2435-2439. DOI:10.1007/s11771-015-2770-9 |
| [9] |
Z.Q. Shi, X.W. Yu, J. Wang, et al., Electrochim. Acta 174 (2015) 215-220. DOI:10.1016/j.electacta.2015.05.133 |
| [10] |
A.K.L. Van, J.K. Mcdonough, S. Li, et al., J. Phys.: Condens. Matter 26 (2014) 284104. DOI:10.1088/0953-8984/26/28/284104 |
| [11] |
M.P. Mousavi, B.E. Wilson, S. Kashefolgheta, et al., ACS Appl. Mater. Interfaces 8 (2016) 3396-3406. DOI:10.1021/acsami.5b11353 |
| [12] |
T. Han, M.S. Park, J. Kim, et al., Chem. Sci. 7 (2015) 1791-1796. |
| [13] |
S. Park, K. Kim, J. Power Source 338 (2017) 129-135. DOI:10.1016/j.jpowsour.2016.10.080 |
| [14] |
H. Wang, L. Pilon, J. Power Source 221 (2013) 252-260. DOI:10.1016/j.jpowsour.2012.08.002 |
| [15] |
D.A.B. Iozzo, M. Tong, G. Wu, et al., J. Phys. Chem. C 119 (2015) 25235-25242. DOI:10.1021/acs.jpcc.5b08409 |
| [16] |
M. Ue, K. Ida, S.J. Mori, Electrochem. Soc. 141 (1994) 2989-2996. DOI:10.1149/1.2059270 |
| [17] |
M. Ue, Electrochemistry 75 (2007) 565-572. DOI:10.5796/electrochemistry.75.565 |
 2019, Vol. 30
2019, Vol. 30 

