b Chongqing Key Laboratory of Soft-Matter Material Chemistry and Function Manufacturing, School of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, China
In the past few decades, many methods were developed for the fabrication of nanomaterials with tunable functionalities by controlling their size, shape, and compositions [1-3]. Morphology-controlled of gold nanooparticles have attracted significant research attention as their optical property, catalytic activity, electrical conductivity and so on [2, 3]. Gold nanoparticle (Au NPs) of various size and shape (such as nanotubes, nanorods, nanocages, nanosheets, nanoflowers) were widely used in fields such as catalysis, sensing, chemical imaging, and biomedicine [2-6].
Thorny nanostructures (also named flower-like, branched, starshaped and urchin-like nanoparticles) have gained more attention [7-11]. The thorny gold nanostructures have exceptionally strong electric field (hot spots) at the tips and gaps between thorns [12-15]. So the thorny nanostructures were successfully employed as SERS substrates for trace toxic ions in biological analysis [6].
At present, the seed-mediated method was often used for synthesizing shaped Au NPs. Several researchers found that Ag+ and organic stabilizers was usually important [11-15]. We recently reported the gold-seed-mediated growth approach to obtain thorny Au NPs, in which the number and length of the thorns could be tailored by introducing different amounts of Ag+ ions into the reaction systems of HAuCl4 and NH2OH in the absence of templates and surfactants [11, 15]. It can be seen that Ag+ has played a very important role in the synthesis of thorny Au NPs.
Based on the above result, a larger number of Ag+ ions were added into the reaction system containing with HAuCl4 and NH2OH·HCl. It was interesting that formed AgCl could induce the growth of thorny Au NPs in the absence of gold seeds, and the morphology of resulted Au NPs was related to the amount of AgNO3. The shape-determined surface-enhanced Raman scattering (SERS) activity of Au NPs was also collected by using rRhodamine 6G (R6G) as Raman probes.
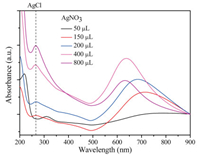
As mentioned above, thorny Au NPs were synthesized by introducing different amount of AgNO3 into the reaction systems of HAuCl4 and NH2OH in the absence of seeds and surfactants. Fig. 1 showed the UV-vis spectra of colloidal Au NPs obtained in the presence of different amount of AgNO3. When a small quantity of AgNO3 (50 μL) was added, there is almost no absorption peak in the long-wave, but there is a weak absorption peak around 310 nm, which belongs to AuCl4- ions, which indicated that HAuCl4 was not reduced. As the amount of AgNO3 increased, a peak of 265 nm appeared gradually, which belongs to AgCl. When the amount of AgNO3 was 150 μL, the SPR peak of Au NPs appeared, indicating that Au NPs were formed. The maximum absorption peak of Au NPs appeared blue shift with the increase of AgNO3.
|
Download:
|
| Fig. 1. UV–vis spectra of Au NPs obtained at different amount of AgNO3. | |
From the XRD pattern (Fig. S1 in Supporting information), it was found that when the amount of AgNO3 was 50 μL and 150 μL, there was no diffraction peak of AgCl. It could be that the quantity of AgCl was below the detection limit of XRD or AgCl with poor crystallinity. The weak diffraction peaks for Au were found at 38° and 44°, corresponding to the Au (111) and (200) planes (JCPDS No. 1-1172). When the amount of AgNO3 was 200 μL or above (400 μL and 800 μL), the peaks at 2θ = 32°, 54°, 57°, and 77°, were assigned as (200), (311), (222), and (420) planes, respectively, of AgCl (JCPDS No. 1-1013). The peaks at 65° and 77° belonged to Au (220) and (311) crystal faces. Fig. S1 indicated that both Au and AgCl existed in the resulted nanoparticles.
It was shown from Fig. 2 that the morphological changes of Au NPs were dependent on the amount of AgNO3. When 150 μL AgNO3 was added, the morphology of the nanoparticles was short-thorny. As the amount of AgNO3 increased to 200 μL, the nanoparticles were long-thorny. Further increasing the amount of AgNO3 to 400 μL, no-thorns Au NPs were generated. If the amount of AgNO3 was 800 μL, the surface roughness of Au NPs decreased, and some of the nanoparticles were hollow structures. In order to confirm the composition of obtained nanoparticles, the sample shown in Fig. 2d was characterized by SEM and EDS (Supporting information). The nanoparticles contained Au, Ag and Cl. The content of Au was the highest, and the atomic percentage of Ag and Cl was about 1:1, which was also confirmed in XRD pattern (Fig. S1). On the other hand, under the same experimental conditions, different amounts of AgNO3 were added to NH2OH·HCl solution. There was only an absorption peak of AgCl, and no absorption peak of Ag, which indicates that NH2OH could not reduce AgNO3 to Ag at this condition (Fig. S3 in Supporting information).

|
Download:
|
| Fig. 2. TEM images of Au NPs at different amount of AgNO3, (a) 150 μL, (b) 200 μL, (c) 400 μL, (d) 800 μL. Scale bars are 200 nm. | |
In short, it could be inferred about the process of forming Au NPs. Firstly; Cl- in reaction system bound Ag+ in AgNO3 to form AgCl. Secondly, colloidal AgCl particles acted as a nucleation center, induce NH2OH·HCl reduce HAuCl4 to Au on its surface, and then regulated the particle morphology through the different amount of AgNO3, which could be explained by the deposition of AgCl precipitates on the surface of gold nanoparticles [11, 15].
It is well known that the rough nanoparticles were excellent substrate for SERS [12-14, 16]. Fig. 3 showed the SERS spectra of R6 G molecules adsorbed on Au NPs, and the comparison of Raman intensity at 1364 cm-1. Whether it was no-thorns, long-thorns, or short-thorns Au NPs, they all exhibited strong Raman enhancement signals. Compared to the no-thorn Au NPs, the Raman signal of Rhodamine from long-thorny Au NPs increased by 1.3 times. The long-thorns Au NPs had the most significant SERS activity, which could be due to the rougher surface and more hot spots. It indicated that the thorny Au NPs had high efficiency as SERS substrate, and might have potential application in SERS based technology.

|
Download:
|
| Fig. 3. (a) SERS spectra of R6 G molecules adsorbed on the Au NPs at different amount of AgNO3, and (b) comparison of Raman intensity at 1364 cm-1. | |
In summary, we have developed a simple, fast, surfactant-free, one-pot method to synthesize thorny Au NPs employing NH2OH·HCl as a reducing agent and AgNO3 as shape-inducing agent. The morphological changes of thorny gold nanoparticle were determined by the amount of Ag+ ions. The thorny Au NPs possessed a strong absorption in the near red region of the visible spectra, and exhibited the good SERS activity, indicating their potential application in analysis and biomedicine.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21277055), and the 111 Project (No. B17019).
Appendix A. Supplementary dataSupplementary material related to this articlecan befound, inthe online version, at doi:https://doi.org/10.1016/j.cclet.2019.01.002.
| [1] |
Y. Xia, X. Xia, H.C. Peng, J. Am. Chem. Soc. 46 (2015) 7947-7966. |
| [2] |
N. Li, P. Zhao, D. Astruc, Angew. Chem. Int. Ed. 53 (2014) 1756-1789. DOI:10.1002/anie.201300441 |
| [3] |
N.N. Jiang, X.L. Zhuo, J.F. Wang, Chem. Rev. 118 (2018) 3054-3099. DOI:10.1021/acs.chemrev.7b00252 |
| [4] |
W. An, Y. Pei, X.C. Zeng, Nano Lett. 8 (2015) 195-202. |
| [5] |
C. Andrea, P. Luana, M. Rita, et al., ACS Nano 9 (2015) 10047-10054. DOI:10.1021/acsnano.5b03624 |
| [6] |
H. Zhao, J. Jin, W. Tian, et al., J. Mater. Chem. A 3 (2015) 4330-4337. DOI:10.1039/C4TA06590E |
| [7] |
L. Zhao, X. Ji, X. Sun, et al., J. Phys. Chem. C 113 (2009) 16645-16651. DOI:10.1021/jp9058406 |
| [8] |
T. Xia, H. Luo, S. Wang, et al., CrystEngComm 17 (2015) 4200-4204. DOI:10.1039/C5CE00407A |
| [9] |
W. Niu, Y.A. Chua, W. Zhang, et al., J. Am. Chem. Soc. 137 (2015) 10460-10463. DOI:10.1021/jacs.5b05321 |
| [10] |
A. Pangdam, K. Wongravee, S. Nootchanat, et al., Mater. Des. 130 (2017) 140-148. DOI:10.1016/j.matdes.2017.05.008 |
| [11] |
H. Yuan, W. Ma, C. Chen, et al., Chem. Mater. 19 (2007) 1592-1600. DOI:10.1021/cm062046i |
| [12] |
E. Hao, R.C. Bailey, G.C. Schatz, et al., Nano Lett. 4 (2015) 71-76. |
| [13] |
M. Yang, R. Alvarez-Puebla, H.S. Kim, et al., Nano Lett. 10 (2010) 4013-4019. DOI:10.1021/nl101946c |
| [14] |
F. Hao, C.L. Nehl, J.H. Hafner, et al., Nano Lett. 7 (2007) 729-732. DOI:10.1021/nl062969c |
| [15] |
H. Yuan, W. Ma, C. Chen, et al., J. Phys. Chem. C 115 (2011) 23256-23260. DOI:10.1021/jp205565y |
| [16] |
C.J. Orendorff, A. Gole, T.K. Sau, C. Murphy, J. Phys. Chem. 109 (2005) 13857-13870. DOI:10.1021/jp0516846 |
 2019, Vol. 30
2019, Vol. 30 

