b Northwest Institute of Nuclear Technology, Xi'an 710024, China
Nowadays, semiconductor metal oxide gas sensors have received extensive attention in the detection and monitoring of harmful gases [1-3]. Acetone, as a good solvent and raw material, has been widely used in organic synthesis in modern industry. But the volatile acetone vapor is harmful to eyes, noses, and central nervous system of human [4]. Besides, acetone is also a breath marker for some diseases such as diabetes [5] and halitosis [6]. Therefore, the detection of acetone with sensor based on oxide semiconductors is of great significance in industrial production, environmental monitoring, detecting toxic gas leakage and disease diagnosis. Studies have suggested that the gas-sensing properties of semiconductor metal oxides are largely related to surface activity of exposed surface. The α-Fe2O3 nanocrystals which were enclosed with facets of high surface energy exhibited excellent catalytic activity and gas-sensing ability [7]. Octahedral nano-SnO2 with high proportion of {221} crystal facets shows better gas sensitivity to alcohols [8]. Tian et al. reported that as the exposure ratio of the {0001} crystal facets of ZnO nanocrystals increased, the response of the sensor material to ethanol was also improved [9].
As an important gas sensing material, titanium dioxide (TiO2) shows the unique advantages of nontoxicity, biocompatibility, low cost, and excellent stability compared with other metal oxides [10-12]. In addition, the resistivity of TiO2 changes significantly with its own structure and surrounding environment [13]. When there is a small amount of oxygen defect in TiO2, its resistivity is several orders of magnitude higher than that of stoichiometric TiO2 [14]. So TiO2 has been considered as one of the important candidates used in the application of photocatalysis [15], photochemistry [16] and gas sensors [17, 18]. The surface structure of nano-TiO2 has also been deeply explored at the atomic level. Theoretical and experimental studies have shown that the {001} crystal facets of the anatase TiO2 has higher surface energy and reactivity than other surfaces. According to the first principle, the {001} crystal facets of TiO2 has more active oxygen adsorption sites, and increasing the exposure ratio of the {001} crystal facets can further improve the gas-sensitivity of TiO2 [19]. Research also shows that {001} surface properties was responsible for the enhanced sensing performance because of its high adsorption activity for active oxygen species [20]. However, the gas sensing properties of TiO2 with defective {001} crystal facets are still in infancy and needs to be explored.
Herein, TiO2 nanoplates with defective and complete {001} crystal facets were prepared by adjusting the concentration ratio of acid in the hydrothermal solution. The sensitivity of complete TiO2 to different gases was tested, and the gas sensitivity of complete TiO2 sensor, defective TiO2 sensor and commercial TiO2 sensor to acetone was further compared, including sensitivity, response-recovery rate and cycle stability. It is proved that the complete high-energy {001} crystal facets play an important role in improving gas-sensing properties of TiO2. We also discussed the corrosion mechanism and gas sensing mechanism of TiO2 nanoplates to gases. This work opens up an encouraging approach to demonstrate the important role of {001} crystal faces in gas sensing.
Different TiO2 samples were synthesized by a hydrothermal method, achieved by adding different concentration of hydrochloric acid. In a typical process, a mixture of hydrofluoric acid and hydrochloric acid solution (30 mL, 19.5 wt%) was stirred at ambient condition for 5 min, and 2.25 mL titanate was added into the solution and stirred for another 5 min. The hydrothermal synthesis was conducted at 180 ℃ for 10 h in a 50 mL Teflon-lined autoclave. After cooling to room temperature, the samples were removed and washed for 5 times with water and ethanol, and then dried in the drying oven at 80 ℃ for 12 h. For the obtained TiO2 with complete {001} crystal facets and defective {001} crystal facets, the volume ratio of hydrofluoric acid to hydrochloric acid was 2/50 and 3/50, respectively (labeled as C-TiO2 and D-TiO2, respectively).
The phases of the samples were characterized by X-ray diffraction analysis (XRD, Cu Kα radiation; λ=1.5408Å). The morphologies were revealed by scanning electron microscope (SEM, Hitachi S-4800) and transmission electron microscopy (TEM, JEM-2100F, 200 kV). The specific surface areas and pore structures measurement were conducted by nitrogen adsorption–desorption experiments (Micromeritics ASAP2020). The X-ray photoelectron spectroscopy (XPS) spectra were examined by an Escalab 250Xi electron spectrometer (Thermo Scientific, USA) using Al Kα radiation. The Brunauer-Emmett-Teller (BET) surface areas of samples were determined by a Micromeritics ASAP 2420 accelerated surface area and porosimetry system. The corresponding pore size distributions were calculated by the Barrett-JoynerHalenda (BJH) method.
The gas sensing test was carried out on the CGS-1TP intelligent gas sensor. First, the obtained sample was mixed with deionized water at a weight ratio of 1:4 to obtain a uniformly paste-like mixture. The paste was coated on the gold electrode with the size of 10 mm × 5 mm × 0.25 mm. Inthispaper, forthe reducinggas, the sensitivity S is the ratio of the resistance of the device in air (Ra) to the resistance in the target gas (Rg), and for the oxidizing gas, S is the ratio of Rg to Ra.
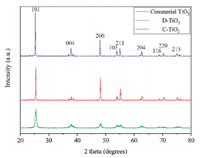
The morphology and structure of the sample were characterized by XRD, SEM and TEM. Fig. 1 shows the XRD patternsof C-TiO2, D-TiO2 and commercial TiO2. The peaks at 25.3°, 35.5°, 37.8°, 38.6°, 48.0°, 53.9°, 55.1°, 62.7°, 68.8°, 70.3° and 75.1° were attributed to the (101), (103), (004), (112), (200), (105), (211), (204), (116), (220) and (215) planes of anatase TiO2 (JCPDF No.21-1272) [21], respectively. All of the three samples were identified to be pure anatase-phase TiO2 [22], and no secondary phases were observed. The difference in the relative broadening of the XRD peaks of the different samples are related to the difference in crystallinity and crystallite sizes [23, 24]. The peaks of C-TiO2 are sharpest compared to the peaks of D-TiO2 and commercial TiO2, indicating that C-TiO2 has the best crystallinity and the smallest half-peak breadth. According to the calculation of Scherrer formula (D=Kγ/Bcosθ), the grain size of C-TiO2 is the largest (41.51 nm) in compared to DTiO2 (33.43 nm) and commercial TiO2 (17.06 nm).
|
Download:
|
| Fig. 1. XRD patterns of C-TiO2, D-TiO2 and commercial TiO2 sensors. | |
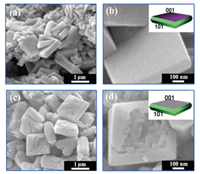
The morphologies of C-TiO2 and D-TiO2 are illustrated in Fig. 2. A plate-like C-TiO2 structure with uniform size can be observed in Fig. 2a. It possesses an average length of 500 nm and an average thickness of 100 nm for C-TiO2 in the enlarged SEM image (Fig. 2b), and the crystal surface of C-TiO2 is very smooth, suggesting that TiO2 with complete crystal facets issynthesized.The insetin Fig. 2b gives a schematic diagram of C-TiO2 structure. On the other hand, excessive HF contributed to the formation of D-TiO2 nanoplates with destroyed surface (Fig. 2c). And many pits can be clearly observed on the {001} crystal facet in the enlarged SEM image (Fig. 2d). The corresponding schematic illustration of D-TiO2 nanoplates is shown in the inset of Fig. 2d.
|
Download:
|
| Fig. 2. (a, c) The SEM images of C-TiO2 and D-TiO2, respectively; (b, d) the corresponding enlarged SEM images. The insets in (b, d) are the schematic illustration. | |
To further confirm the microstructures characteristics of the received TiO2, we recorded HR-TEM images of C-TiO2 and D-TiO2 (Fig. 3). The HR-TEM image of TiO2 clearly showed lattice fringe with an interplanar distance of 0.235 nm (Fig. 3a), suggesting the existence of high-energy {001} crystal facets of anatase-phase TiO2 [25, 26]. At the edge of TiO2 nanoplates, the crystal fringe of anatase TiO2 (101) facets with an interplanar distance of 0.351 nm could be seen (Fig. 3b), confirming that C-TiO2 mainly consists of {001} crystal facets and also contains a small portion of {101} crystal facets [27]. Meanwhile, the continuous lattice lines indicate that prepared samples are well-crystallized C-TiO2. As can be seen from Fig. 3c, the surface of the crystal is uneven and partially destroyed (the region marked by red curves), which reveals that the generation of lattice defects. The interplanar spacing of the undamaged part is 0.235 nm, corresponding to {001} crystal facets. The discontinuity of the lattice means that the {101} crystal facets were dissolved by excessive HF.

|
Download:
|
| Fig. 3. HR-TEM images of TiO2: (a) {001} facet of C-TiO2, (b) {101} facet of C-TiO2, (c) {001} facets of D-TiO2. | |
During the synthesis of TiO2, the introduction of morphology control agent F- greatly reduces the surface energy of the {001} crystal plane and promotes the growth of the {001} crystal plane, thereby obtaining an anatase structure TiO2 with a high proportion of {001} crystal planes. However, after the addition of excessive HF, the acid reacts with TiO2 and the reaction produces a water-soluble ion TiF62-, resulting in chemical dissolution of TiO2 [28]. The reaction equation is as follows:
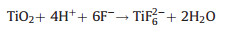
|
(1) |
Fig. S1 (Supporting information) shows the XPS test was also employed to investigate the surface chemical composition of C-TiO2 and D-TiO2. As can be seen from Figs. S1a and b, the ratio of Ti element to O element in both samples is about 1:2, confirming that the composition of the two samples are TiO2. In Fig. S1c, two peaks can be seen at 464.6 eV and 458.9 eV, which belong to Ti 2p1/2 and Ti 2p3/2 orbits, respectively [29]. This indicates that the Ti element in the synthesized sample exists mainly in the form of Ti4+. However, the position of the peaks in Fig. S1d is slightly offset (0.2 eV) from the peaks in Fig. S1c (464.8 eV and 459.1 eV, respectively), which may be caused by the corrosion of the crystal facets.
It is reported that the gas-sensing properties are very sensitive to the specific surface area and pore structure of the sensing material [26, 27]. Thus, nitrogen adsorption-desorption measurements were performed on C-TiO2 and D-TiO2 (Fig. S2 in Supporting information). The BJH analyses (the inset of Fig. S2a) showed that the commercial TiO2 exhibited a pore size-distribution of 20 nm calculated from the desorption branch of the nitrogen adsorption isotherm. The results showed that the commercial TiO2 has a high BET specific surface area of 102.58 m2/g. But the calculated surface areas of complete {001} surfaces and defective {001} surfaces are 1.47 m2/g and 1.54 m2/g, respectively, which are far less than the surface area of commercial TiO2. Moreover, the corresponding pore diameters of the two samples (8.21 nm and 7.86 nm) are also much smaller compared to commercial TiO2, which is due to the fact that the particle size of commercial TiO2 is smaller than the particle size of the C-TiO2 and D-TiO2.
Next, we explored the gas sensitivity of the sample, including gas sensitivity selectivity, sensitivity and cyclic stability. Selectivity is a very important parameter for gas sensors. Fig. 4a shows the sensitivity of the prepared C-TiO2 to several different gases with a concentration of 100 ppm at 250~500 ℃. The sensitivities of the C-TiO2 to benzene, acetone, toluene, methanol, ethanol, and isopropanol at 400 ℃ are 2.73, 21.8, 4.32, 4.08, 8.46, and 6.64, respectively. It is clear that the C-TiO2 has the highest response to acetone (21.8), which is 2.8 times greater than that of the second highest sensitive gas (ethanol). In other words, C-TiO2 exhibits excellent gas-sensitive selectivity to acetone. In order to investigate the optimum sensing temperature, the sensitivity of C-TiO2, D-TiO2 and commercial TiO2 sensors at different temperatures (250~500 ℃) to 100 ppm acetone was tested. As shown in the Fig. 4b, it is found that all of the response curves exhibited a "parabola" shape as the working temperatures increased, and the optimum temperature is 400 ℃. Notably, the maximum response values of the C-TiO2 sensor is 21.8, which is about 1.5 and 5.3 times greater than that of the D-TiO2 (14.5) and commercial TiO2 (4.8), respectively.

|
Download:
|
| Fig. 4. (a) Sensitivity of the C-TiO2 sensors to different gases at different operating temperatures; (b) Line chart of sensitivity of C-TiO2, D-TiO2 and commercial TiO2 sensors to 100 ppm acetone at different operating temperatures; (c) The sensing responses of the commercial TiO2, C-TiO2 and D-TiO2 sensors toward acetone with various concentrations at 400 ℃, and (d) the corresponding line chart. | |
When the sensitivity is lower than the optimum temperature, the energy of the system cannot overcome the activation energy, resulting in low sensitivity response. When the temperature is higher than the optimum sensing temperature, a lot of heat will accumulate on TiO2 nanoplates surface and accelerates the desorption of target gas molecules adsorbed on TiO2 nanoplates surface before reaction, resulting in reducing sensitivity of the sensor. From Fig. 4b, it is obvious that the gas sensitivity of C-TiO2 is much higher than D-TiO2 and commercial TiO2 sensor, which indicates that TiO2 nanoplates with complete {001} facets has higher gas sensing activity in comparison to TiO2 nanoplates with defective {001} facets and TiO2 powder.
The sensitivity of the sensor to different concentration reflects the ability of sensor to distinguish the different concentrations of gas, and it is also an important physical parameter in the practical application. Fig. 4c shows the response characteristics of C-TiO2 and D-TiO2 sensors to 10~200 ppm acetone at 400 ℃. It can be seen that sensitivity of two sensors to acetone increases with the increment of gas concentration, which means that the sensitivity of C-TiO2 and D-TiO2 to acetone is positively related to the gas concentration. The continuous response of the sensors to different concentrations of gas indicates that the sensor can continuously detect target gas molecules within the applicable temperature range.
The sensitivity curves of different concentration of acetone were plotted to further study the relationship between sensitivity and gas concentration (Fig. 4d). It can be found that the response of the three sensors increase with acetone concentration at the optimal working temperature, and the C-TiO2 sensor shows the greatest increase in response. The response curves are nearly linear at low acetone concentrations (10~200 ppm) and increase slowly at high acetone concentrations due to the saturation of the sensors [30, 31]. The linear relationship at low concentrations lays the foundation for quantitative measurements in the future.
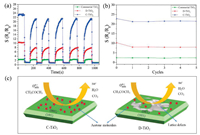
In order to investigate the reproducibility of three TiO2 sensors, the dynamic response properties under 100 ppm acetone were represented in Fig. 5a. It can be seen that the resistance of C-TiO2 sensor can recover to the former value when the sensor is exposed to the air again and no obvious baseline drift can be observed. The corresponding line chart (Fig. 5b) also clearly reveals that the sensitivity of C-TiO2 sensor to acetone is almost unchanged after 5 cycles, indicating that C-TiO2 sensor exhibits good reproducibility.
|
Download:
|
| Fig. 5. (a) Sensing reproducibility of the commercial TiO2, C-TiO2 and D-TiO2 toward 100 ppm acetone at 400 ℃, and (b) the corresponding line chart; (c) Schematic diagram of the gas-sensing mechanism of C-TiO2 and D-TiO2 sensor to acetone. | |
In order to better explain the differences in gas sensitivity between C-TiO2 and D-TiO2, the gas sensitivity mechanism is proposed as follows: In an air atmosphere, oxygen molecules adsorb on the surface of n-type TiO2 to produce oxygen species adsorbate (O2-, O2-, O-), forming an electron depletion region and reducing the conductivity of the sensor [32, 33]. When TiO2 is exposed to acetone gas, reducing gas acetone will react with the anionic oxygen absorbed on the surface, and electrons are injected into the conduction band of TiO2 (Fig. 5c). The injection of electrons will reduce the electron depletion region and increase the concentration of free carriers, resulting in a decrease in the conductivity of the sensor. The reaction equations are as follows:
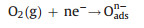
|
(2) |
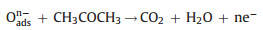
|
(3) |
It is generally believed that the gas sensing properties of semiconducting oxides mainly depend on the properties of crystal facets [34]. In this study, C-TiO2 shows enhanced gas-sensing properties in compared to D-TiO2, which may be attributed to the more acetone molecular adsorbed on the complete {001} facets to react with oxygen species (O2-, O2-, O-). It has been reported that the acetone molecules are much more favorable to be adsorbed on the {001} facets than on other facets due to the much lower adsorption energy of the acetone molecule on the {001} surface [35, 36]. In the case of D-TiO2, less acetone molecules will be adsorbed on the {001} crystal facets due to the destruction of the {001} facets. Meanwhile, the destruction of {001} crystal facet in D-TiO2 has produced many lattice defects which will hinder the electron transfer. Thus, there is insignificant change in the resistivity of D-TiO2 sensor. As a result, the D-TiO2 showed a much lower response toward acetone than that of C-TiO2. It should be also noted that C-TiO2 with the lowest specific surface area shows the highest gas response in comparison to D-TiO2 and commercial TiO2 sensor, further confirming the high-energy {001} crystals of C-TiO2 play an important role in improving gas-sensing performance.
In summary, TiO2 nanoplates with complete {001} and defective {001} crystal facets have been prepared and characterized. The gas-sensing properties of C-TiO2 and D-TiO2 along with the commercial TiO2 to acetone were carefully studied and discussed. The results show that the sensing response of C-TiO2 is higher than those of D-TiO2 and commercial TiO2, indicating that the crystal surface property is the main factor that affects the gassensitivity of TiO2, rather than the specific surface area. Furthermore, compared to C-TiO2, the gas sensitivity of D-TiO2 sensor is significantly reduced, which also offers an evidence that the highenergy crystal {001} plane plays an important role in improving the gas sensitive properties of TiO2. The good performance of {001} crystal facets is contributed to its high adsorption capacity to acetone and rapid transfer of electrons. And exposure of the highenergy {001} crystal surface also provides a feasible method for improving the sensitivity and selectivity of the TiO2 sensors.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 51773226, 61701514), the Natural Science Foundation of Hunan Province (No. 2018JJ3603).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.12.030.
| [1] |
X. Zhou, X. Cheng, Y. Zhu, et al., Chin. Chem. Lett. 29 (2018) 405-416. DOI:10.1016/j.cclet.2017.06.021 |
| [2] |
Q. Zhang, H. Zhang, M. Xu, Z. Shen, Q. Wei, Chin. Chem. Lett. 29 (2018) 538-542. DOI:10.1016/j.cclet.2017.09.018 |
| [3] |
B. Wang, Y.D. Wang, Y.P. Lei, et al., J. Mater. Chem. C 4 (2016) 295-304. DOI:10.1039/C5TC02792F |
| [4] |
Q. Jia, H. Ji, Y. Zhang, et al., J. Hazard. Mater. 276 (2014) 262-270. DOI:10.1016/j.jhazmat.2014.05.044 |
| [5] |
B.Q. Wang, Q. Yu, S.F. Zhang, et al., Sensor. Actuat. B -Chem. 258 (2018) 1215-1222. DOI:10.1016/j.snb.2017.12.018 |
| [6] |
D. Zhang, A. Liu, H. Chang, B. Xia, RSC Adv. 5 (2015) 3016-3022. DOI:10.1039/C4RA10942B |
| [7] |
J.J. Ouyang, J. Pei, Q. Kuang, Z.X. Xie, L.S. Zheng, ACS Appl. Mater. Interfaces 6 (2014) 12505-12514. DOI:10.1021/am502358g |
| [8] |
W. Zeng, T. Liu, Z.C. Wang, et al., J. Mater. Sci.-Mater. Electron. 51 (2010) 171-175. |
| [9] |
Z.H. Wang, J. Xue, D.M. Han, F. Gu, ACS Appl. Mater. 7 (2015) 308-317. DOI:10.1021/am506206c |
| [10] |
J. Bai, B.X. Zhou, Chem. Rev. 114 (2014) 10131-10176. DOI:10.1021/cr400625j |
| [11] |
L. Liu, X.M. Yu, B. Zhang, S.X. Meng, Y.Q. Feng, Chin. Chen. Lett. 28 (2017) 765-770. DOI:10.1016/j.cclet.2017.03.011 |
| [12] |
G.L. Liu, C. Han, M. Pelaez, et al., Nanotechnology 23 (2012) 294003. DOI:10.1088/0957-4484/23/29/294003 |
| [13] |
D. Buso, M. Post, C. Cantalini, P. Mulvaney, A. Martucci, Adv. Funct. Mater. 18 (2008) 3843-3849. DOI:10.1002/adfm.v18:23 |
| [14] |
C.C. Lu, Y.S. Huang, J.W. Huang, C.K. Chang, S.P. Wu, Sensors 10 (2010) 670-683. DOI:10.3390/s100100670 |
| [15] |
C. Han, Y. Wang, Y. Lei, et al., Nano Res. 8 (2015) 1199-1209. DOI:10.1007/s12274-014-0600-2 |
| [16] |
J. Song, J. Wang, X. Lin, Z. Chu, et al., ChemElectroChem. 4 (2017) 514-520. DOI:10.1002/celc.v4.3 |
| [17] |
B. Wang, L. Deng, L. Sun, et al., Sensor. Actuat. B -Chem. 276 (2018) 57-64. DOI:10.1016/j.snb.2018.08.080 |
| [18] |
Y.L. Wang, S. Tan, J. Wang, et al., Chin. Chem. Lett. 22 (2011) 603-606. DOI:10.1016/j.cclet.2010.11.020 |
| [19] |
W. Zeng, T. Liu, Z.C. Wang, et al., Mater. Trans. 51 (2010) 171-175. DOI:10.2320/matertrans.M2009317 |
| [20] |
X.G. Han, M.S. Jin, S.F. Xie, et al., Chem. Int. Ed. 48 (2009) 9180-9183. DOI:10.1002/anie.v48:48 |
| [21] |
H. Labiadh, T.B. Chaabane, L. Balan, et al., Appl. Catal. B: Environ. 144 (2014) 29-35. DOI:10.1016/j.apcatb.2013.07.004 |
| [22] |
Y. Ding, Y. Wang, L. Zhang, Nanoscale 3 (2011) 1149-1157. DOI:10.1039/c0nr00773k |
| [23] |
M.M. Arafat, A.S.M.A. Haseeb, S.A. Akbar, Sensors 14 (2014) 13613-13627. DOI:10.3390/s140813613 |
| [24] |
C.X. Wang, L.W. Yin, L.Y. Zhang, et al., Langmuir 26 (2010) 12841-12848. DOI:10.1021/la100910u |
| [25] |
J. Pan, G. Liu, G.Q. Lu, et al., Chem. Commun. 50 (2011) 2133-2137. |
| [26] |
Y. Yang, G.Z. Wang, Q. Deng, et al., RSC Adv. 4 (2014) 34577-34583. DOI:10.1039/C4RA04787G |
| [27] |
Y. Yang, G.Z. Wang, Q. Deng, D.H.L. Ng, H.J. Zhao, ACS Appl. Mater. Interfaces 6 (2014) 3008-3015. DOI:10.1021/am405607h |
| [28] |
W.X. Guo, C. Xu, X. Wang, et al., J. Am. Chem. Soc. 134 (2012) 4437-4441. DOI:10.1021/ja2120585 |
| [29] |
X.H. Liu, J. Zhang, S.H. Wu, et al., RSC Adv. 2 (2012) 6178-6184. DOI:10.1039/c2ra20797d |
| [30] |
Z. Lou, F. Li, J.A. Deng, L.L. Wang, T. Zhang, ACS Appl. Mater. Interfaces 5 (2013) 12310-12316. DOI:10.1021/am402532v |
| [31] |
J.A. Deng, L.L. Wang, Z. Lou, T. Zhang, J. Mater. Chem. A 2 (2014) 9030-9034. DOI:10.1039/C4TA00160E |
| [32] |
X.R. Zhou, X.W. Cheng, Y.H. Zhu, et al., Chin. Chen. Lett. 29 (2017) 405-416. |
| [33] |
D. Liu, L. Lin, Q. Chen, H. Zhou, J. Wu, ACS Sens. 2 (2017) 1491-1497. DOI:10.1021/acssensors.7b00459 |
| [34] |
P.Q. Hu, G.J. Du, W.J. Zhou, et al., ACS Appl. Mater. Interfaces 2 (2010) 3263-3269. DOI:10.1021/am100707h |
| [35] |
Y. Yong, L. Yan, G.Z. Wang, et al., ACS Appl. Mater. Interfaces 7 (2015) 24902-24908. DOI:10.1021/acsami.5b08372 |
| [36] |
L.L. Liu, Q. Liu, Y.P. Zheng, et al., J. Phys. Chem. C 118 (2014) 3471-3482. DOI:10.1021/jp408221x |
 2019, Vol. 30
2019, Vol. 30 

