b Department of Chemistry, Hunan University of Science and Engineering, Yongzhou 425100, China;
c School of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China;
d Hunan Provincial Key Laboratory of Materials Protection for Electric Power and Transportation, Changsha University of Science and Technology, Changsha 410114, China
In green and sustainable chemistry, it has been under active pursuit to develop protocols that can minimize the chemical waste, in the meanwhile maximize the sustainability of synthetic reactions [1]. Most of the high-value-added fine chemicals and synthetic drugs are produced in the presence of organic volatile compounds as reaction media, accompanied by the generation of unwanted by-products and/or unconsumed raw materials, which not only cause cumbersome purification process and serious environmental pollution but also lead to high manufacturing cost of the terminal products. As a consequence, in both academia and chemical industry, there has been a pressing need for the development of highly efficient synthetic reactions and the substitution of conventional organic solvents with environment friendly solvents (such as biomass solvents [2], water [3], ionic liquids [4], supercritical CO2 [5]) in chemical transformations. Although a lot of synthetic reactions in various eco-friendly solvents have been well established with decent yields [6], the green separation and purification still represents a critical challenge because traditional post-treatment process often consumes a large amount of petroleum-derived solvents, which inevitably causes subsequent environment pollution as well as higher production costs. For example, the vast disposal cost for metal-free, nonhazardous organic solid ranges from $700 to $950 per ton in China, and the cost of organic solvents have been estimated at four billion pounds annually in the pharmaceutical industry [7]. Therefore, an actual challenge in green organic synthesis is to develop highly efficient and green synthetic reaction with sustainable separation and purification procedures.
Z-3-Selenocyanatoacrylate is one of the most important selenium-containing compounds [8], given the widespread application of selenocyanate [9] and acrylate [10] groups in both organic synthesis and pharmaceutical chemistry [11]. Our group has reported the synthesis of Z-3-selenocyanatoacrylates through deep eutectic ChCl/glycolic acid (molar ratio 1:2) catalyzed multicomponent reaction [12] of alkynoates [13], potassium selenocyanate (KSeCN) and water in the presence of as the catalyst and reaction media [14]. However, mixtures of stereoisomers are produced in all cases and the deep eutectic solvents are not commercially available. Furthermore, the protocol did not solve the post-treatment problem, since the final work-up and purification process employing a large amount of volatile organic compounds is required to collect the pure products from the mixtures of stereoisomers and unconsumed alkynoates. It is therefore not surprising that a green and sustainable process for Z-3-selenocyanatoacrylates formation and post-treatment has been highlighted as a significant challenge in both academic and industrial research.
Over the past few years, we have put effort into developing green organic synthesis [15]. Herein, we report a sustainable protocol for the preparation of various Z-3-selenocyanatoacrylates by using lactic acid as a reusable catalyst and reaction media. In this process, the generation of chemical waste in the traditional synthesis and purification of Z-3-selenocyanatoacrylates can be completely circumvented.
Our initial efforts focused on optimizing the reaction conditions for the construction of (Z)-ethyl 3-selenocyanatoacrylate 2a from ethylpropiolate 1a and KSeCN.Treatment of a mixture of 1a, KSeCN (1.2 equiv.) and lactic acid (4 equiv.) at ambient temperature under ultrasonic conditions (30 W/44 KHz/25–32 ℃) in open air for 15 min led to a quantitative selenocyanation yield (98%) of the expected product 2a and full consumption of 1a (Table 1, entry 1).No propiolic acid (in situ generated from the hydrolysis of ethyl propiolate) was detected by HRMS.When other biomass acids were employed as the reaction media, 2a was generated in lower yields and moderate stereoselectivities (entries 2–9). Varying the ratio of KSeCN and 1a revealed that a slight excess of KSeCN was needed to ensure complete conversion of 1a (entry 10). Reducing the loadingof lactic acid diminished the reaction yield (entry 11). Performing the reaction with lactic acid aqueous (1 mol/L, 1 mL) gave much lower yield of 2a (entry 12). When mechanical grinding or stirring was used instead of ultrasonic radiation, a lower yield of 2a was observed (entries 13 and 14). No selenocyanation reaction took place in a blank test under the standard reaction conditions (entry 15). Thus, the optimal conditions are: ethyl propiolate (1a), KSeCN(1.2 equiv.), lacticacid (4 equiv.), USI(30 W/44 kHz/25-32 ℃)for 15 minin open air conditions (Scheme 1).
|
|
Table 1 Optimization of reaction conditions.a |

|
Download:
|
| Scheme 1. Synthetic route to sulfonylated quinolines. | |
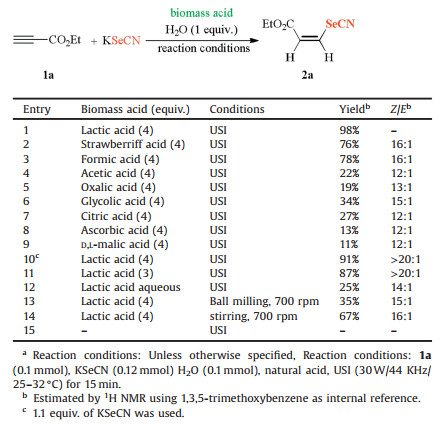
With the optimal reaction conditions in hand, we then investigated the scope of this sustainable selenocyanation reaction. As shown in Scheme 2, all the tested activated alkynes showed high reactivity delivering full conversions and the stereospecific products were formation in excellent yields. A series of structurally diverse terminal and internal alkynoates efficiently reacted with KSeCN via lactic acid-catalyzed selenocyanation reactions to afford Z-3-selenocyanatoacrylates in excellent yields of 90%-96% (2a-2r). Their chromatographic data and chromatography are deposited in Supporting information. Activated alkynes containing bioactive natural alcohols, such as piperonyl alcohol, perill alcohol, and cinnamyl alcohol, still delivered the corresponding products (2p–2r) in excellent yields. In addition, various electron-poor substituted acetylenes, ynone (1s), propargylic thioesters (1t–1u) and proparagyl sulfonate (1v), could also be effciently converted into the expected Z-β-vinyl selenolates. To obtain the pure selenocyanation products, the deep eutectic ChCl/glycolic acid (molar ratio 1:2)-promoted selenocyanation reaction requires both volatile organic solvent extraction and column chromatographic purification, resulting in the inevitable formation of large amounts of chemical waste during the post-process procedure. The insolubility of selenocyanation products in lactic acid allows their facile isolation and purification through simple extraction without traditional silica gel chromatographic purification in the sub-gram scale preparation (Condition A). Significantly, in the 5 mmol selenocyanation of alkynoates (1a–1i), all the desired products were synthesized with almost quantitative yield in the presence of 3 equiv. of lactic acid and the pure products were collected through water precipitation method (Condition B).
|
Download:
|
| Scheme 2. Scope study of alkynes. Reaction conditions A: 1 (0.2 mmol), KSeCN (0.24 mmol), H2O (0.2 mmo), lactic acid (0.8 mmol), USI (30 W/44 KHz). Reaction conditions B: 1 (5 mmol), KSeCN (6 mmol), H2O (5 mmo), lactic acid (15 mmol), USI (30 W/44 KHz/25-32 oC) for 30 min. Isolated yield. | |
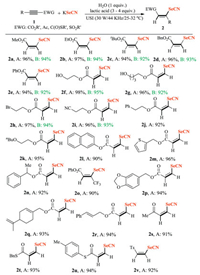
The large-scale preparation and the reusability of reaction media are of great importance in fine chemistry and medicine industry. To further demonstrate the scalability of the present transformation, the template selenocyanation reaction of ethyl propiolate was scaled up to 15 and 30 mmol, and 2.8 and 5.5 g of (Z)-ethyl 3-selenocyanatoacrylate 2a was easily obtained, respectively (Scheme 3).
|
Download:
|
| Scheme 3. Large-scale synthesis and 1H NMR of 2a. | |
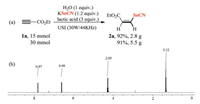
In order to prove the reusability of the biomass reaction system, the lactic acid-catalyzed selenocyanation reaction of ethyl propiolate was chosen as a template reaction. After completion of the first reaction, the targeted product 2a was collected via water precipitation method. The biomass lactic acid was directly employed for next cycle. As shown in Fig. 1, the cheap biomass lactic acid could be re-used without obvious decrease in catalytic activity during five consecutive runs.
|
Download:
|
| Fig. 1. Reusability of lactic acid. | |
The present report highlights the establishment of a sustainable biomass reaction system for the preparation of Z-3-selenocyanatoacrylates and analogues. A significant advantage of this developed protocol is organic volatile compounds can be avoided entirely in the synthesis, work-up and purification processes, as the conversion of the activated alkynes into the desired products is almost quantitative or quantitative with a minimal amount of lactic acid employed as the reaction media, and the pure products can be collected through water precipitation. Furthermore, the cheap lactic acid could be easily recovered and showed high catalytic activity within five successive reaction runs. This developed strategy was highly attractive in the field of eco-friendly synthetic chemistry and for the green pharmaceutical industry.
AcknowledgmentWe are grateful for financial support from the Natural Science Foundation of Hunan Provincial (Nos. 2019JJ50193 and 2019JJ20008).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2019.04.033.
| [1] |
(a) M. He, B. Han, Sci. China Chem. 60 (2017) 837-838; (b) B. Han, Acta Phys. Chim. Sin. 34 (2018) 837-837; (c) S. Hao, L.X. Li, D.Q. Dong, Z.L. Wang, X.Y. Yu, Tetrahedron Lett. 59 (2018) 4073-4075; (d) G.H. Li, D.Q. Dong, X.Y. Yu, Z.L. Wang, New J. Chem. 43 (2019) 1667-1670; (e) Q. Liu, L. Wang, H. Yue, et al., Green Chem. 21 (2019) 1609-1603; (f) J. Xu, W. Huang, R. Bai, et al., Green Chem. 21 (2019) 2061-2069. |
| [2] |
(a) J. Yang, J.N. Tan, Y. Gu, Green Chem. 14 (2012) 3304-3317; (b) Y. Gu, F. Jerome, Chem. Soc. Rev. 42 (2013) 9550-9570. |
| [3] |
(a) M. Salehpour, J. Azizian, H. Kefayati, Chin. Chem. Lett. 28 (2017) 1079-1082; (b) Z. Chen, X.X. Shi, D.Q. Ge, et al., Chin. Chem. Lett. 28 (2017) 231-234; (c) D.Q. Dong, S.H. Hao, H. Zhang, Z.L. Wang, Chin. Chem. Lett. 28 (2017) 1597-1599; (d) W. Wei, P. Bao, H. Yue, et al., Org. Lett. 20 (2018) 5291-5295; (e) J. Yang, F. Mei, S. Fu, Y. Gu, Green Chem. 20 (2018) 1367-1374; (f) L. Liu, Q. Sun, Z. Yan, et al., Green Chem. 20 (2018) 3927-3930; (g) D. Chen, Y. Zhang, X. Pan, F. Wang, S. Huang, Adv. Synth. Catal. 360 (2018) 3607-3612; (h) Y. Huo, P. Shen, W. Duan, et al., Chin. Chem. Lett. 29 (2018) 1359-1362; (i) J. Li, W. Tang, D. Ren, J. Xu, Z. Yang, Green Chem. 21 (2019) 2088-2094. |
| [4] |
(a) S.B. Yu, H.J. Zang, X.L. Yang, et al., Chin. Chem. Lett. 28 (2017) 1479-1484; (b) X.Y. Li, S.S. Zheng, X.F. Liu, et al., ACS Sustainable Chem. Eng. 6 (2018) 8130-8135; (c) B. Lai, R. Bai, Y. Gu, ACS Sustainable Chem. Eng. 6 (2018) 17076-17086; (d) D.Q. Dong, W.J. Chen, Y. Yang, X. Gao, Z.L. Wang, ChemistrySelect 4 (2019) 2480-2483. |
| [5] |
F.Q. Meng, X.J. Feng, W.H. Wang, M. Bao, Chin. Chem. Lett. 28 (2017) 900-904. DOI:10.1016/j.cclet.2016.12.018 |
| [6] |
(a) Y. Han, M. Zhang, Y.Q. Zhang, Z.H. Zhang, Green Chem. 20 (2018) 4891-4900; (b) L. Wang, D. Xiong, L. Jie, C. Yu, X. Cui, Chin. Chem. Lett. 29 (2018) 907-910. |
| [7] |
H.C. Hailes, Org. Process Res. Dev. 11 (2007) 114-120. DOI:10.1021/op060157x |
| [8] |
(a) T. Guo, X.N. Wei, H.Y. Wang, et al., Org. Biomol. Chem.15 (2017) 9455-9464; (b) T. Guo, X.N. Wei, Y. Liu, P.K. Zhang, Y.H. Zhao, Org. Chem. Front. (2019), doi: http://dx.doi.org/10.1039/C9QO00198K; (c) K. Sun, S. Wang, R. Feng, et al., Org. Lett. 21 (2019) 2052-2055; (d) H. Jiang, X. Tang, Z. Xu, et al., Org. Biomol. Chem. 17 (2019) 2715-2720. |
| [9] |
(a) K. Sun, Y. Lv, Z. Shi, et al., Sci. China Chem. 60 (2017) 730-733; (b) K. Sun, X. Wang, F. Fu, et al., Green Chem. 19 (2017) 1490-1493; (c) L.H. Lu, S.J. Zhou, W.B. He, et al., Org. Biomol. Chem. 16 (2018) 9064-9068. |
| [10] |
(a) Y. Yang, L. Tang, S. Zhang, et al., Green Chem. 16 (2014) 4106-4109; (b) X. Fu, Y. Meng, X. Li, M. Stępien, P.J. Chmielewski, Chem. Commun. 54 (2018) 2510-2513; (c) D. Ren, B. Liu, X. Li, et al., Org. Chem. Front. 6 (2019) 908-918. |
| [11] |
(a) W. Xie, S. Xie, Y. Zhou, et al., Eur. J. Med. Chem. 81 (2014) 22-27; (b) W. Xie, H. Zhang, J. He, et al., Bioorg. Med. Chem. Lett. 27 (2017) 530-532; (c) W. Xie, Y. Wu, J. Zhang, et al., Eur. J. Med. Chem. 145 (2018) 35-40; (d) Q. Liang, Y. Zhang, M. Zeng, et al., Toxicol. Res. 7 (2018) 521-528; (e) Q. Liang, Y. Zhang, M. Huang, Y. Xiao, F. Xiao, Mol. Med. Rep. 19 (2019) 1256-1265; (f) Y. Xiao, M. Zeng, L. Yin, N. Li, F. Xiao, Toxicol. Res. 8 (2019) 15-24; (g) G.H. Li, D.Q. Dong, Y. Yang, X.Y. Yu, Z.L. Wang, Adv. Synth. Catal. 361 (2019) 832-835. |
| [12] |
(a) X. Gong, J. Chen, X. Li, W. Xie, J. Wu, Chem. Asian J. 13 (2018) 2543-2548; (b) Y. Gu, W. Huang, S. Chen, X. Wang, Org. Lett. 20 (2018) 4285-4289; (c) K. Sun, Z. Shi, Z. Liu, et al., Org. Lett. 20 (2018) 6687-6690; (d) Y.H. Wang, B. Ouyang, G. Qiu, H. Zhou, J.B. Liu, Org. Biomol. Chem. (2019), doi: http://dx.doi.org/10.1039/C9OB00320G; (e) Y. Zong, Y. Lang, M. Yang, et al., Org. Lett. 21 (2019) 1935-1938; (f) S. Ye, X. Li, W. Xie, J. Wu, Asian J. Org. Chem. (2019), doi: http://dx.doi.org/10.1002/ajoc.201900172; (g) Y. Zhang, K. Sun, Q. Lv, et al., Chin. Chem. Lett. (2019), doi: http://dx.doi.org/10.1016/j.cclet.2019.03.034; (h) T. Yuan, C. Pi, C. You, et al., Chem. Commun. 55 (2019) 163-166; (i) G. Li, Z. Gan, K. Kong, X. Dou, D. Yang, Adv. Synth. Catal. 361 (2019) 1808-1814; (j) Z. Li, C. Fang, Y. Zheng, et al., Tetrahedron Lett. 59 (2018) 3934-3937; (k)G.H.Li, D.Q.Dong, Q.Deng, S.Q.Yan, Z.L.Wang, Synthesis(2019), doi: http://dx.doi.org/10.1055/s-0037-1611787. |
| [13] |
(a) G.P. Lu, C. Cai, F. Chen, R.L. Ye, B.J. Zhou, ACS Sustainable Chem. Eng. 4 (2016) 1804-1809; (b) W. Wei, L. Wang, H. Yue, Y.Y. Jiang, D. Yang, Org. Biomol. Chem. 16 (2018) 7001-7005; (c) Z. Yang, L. Jie, Z. Yao, Z. Yang, X. Cui, Adv. Synth. Catal. 361 (2019) 214-218. |
| [14] |
C. Wu, H.J. Xiao, S.W. Wang, et al., ACS Sustainable Chem. Eng. 7 (2019) 2169-2175. DOI:10.1021/acssuschemeng.8b04877 |
| [15] |
(a) L.Y. Xie, Y.J. Li, J. Qu, et al., Green Chem. 19 (2017) 5642-5646; (b) L.Y. Xie, Y. Duan, L.H. Lu, et al., ACS Sustainable Chem. Eng. 5 (2017) 10407-10412; (c)L.Y. Xie, S. Peng, J.X. Tan, et al., ACS Sustainable Chem. Eng. 6 (2018) 16976-16981; (d) W.H. Bao, C. Wu, J.T. Wang, et al., Org. Biomol. Chem. 16 (2018) 8403-8407; (e) C. Wu, J. Wang, X.Y. Zhang, et al., Org. Biomol. Chem. 16 (2018) 5050-5054; (f) Z. Wang, L. Yang, H.L. Liu, et al., Chin. J. Org. Chem. 38 (2018) 2639-2647; (g) Y. Sheng, Y. You, Z. Cao, L. Liu, H.C. Wu, Analyst 143 (2018) 2411-2415; (h) L.Y. Xie, S. Peng, T.G. Fan, et al., Sci. China Chem. 62 (2019) 460-464; (i) Y. You, K. Zhou, B. Guo, et al., ACS Sens. 4 (2019) 774-779; (j) Z. Cao, W.F. Li, C. Liu, et al., Chin. J. Anal. Chem. 47 (2019) 229-236; 8798-8803; (l) L.Y. Xie, S. Peng, F. Liu, et al., ACS Sustainable Chem. Eng. 7 (2019) 7193-7199; (m) T.Y. Shang, L.H. Lu, Z. Cao, et al., Chem. Commun. (2019), doi: http://dx.doi.org/10.1039/C9CC01047E. |
 2019, Vol. 30
2019, Vol. 30