b College of Environment Sciences, Sichuan Agricultural University, Chengdu 611130, China
As the byproduct of rice harvest, rice straw (RS) is the most abundant crop straw in China, with approximately 0.3 billion RS generated each year [1]. Converting RS to biogas with anaerobic digestion technology is considered as one of the most ecofriendly and effective ways to treat agricultural waste and develop clean energy [2]. RS is a lignocellulosic material, mainly consisting of three types of polymers: cellulose, hemicellulose, and lignin. However, only cellulose and hemicellulose can be hydrolyzed into monosaccharides or simple sugars, and subsequently converted into biogas [3]. The cellulose connects each other with hemicellulose and amorphous polymers of different sugars, which are overlapped by lignin [4]. The lignocarbohydrate complexes form strong bonds, which significantly limit the hydrolysis rate [5]. Furthermore, lignin, which is insoluble in water and highly resistant to anaerobic microbial attack and normal fermentation technology, is considered to be the major barrier of lignocellulosic degradation [6, 7]. Therefore, breaking down or dissolving the lignin component through some pretreatment measures is believed to be a feasible method to accelerate hydrolysis and biogas production rates [4]. However, reports on cost-effective RS pretreatment processes for commercial operation are limited [8]. The existing pretreatment methods mainly include mechanical, chemical, and biological processes, which may not be economically feasible for practical applications. For instance, mechanical pretreatment require high energy inputs, chemical pretreatment may cause re-contamination owing to additives, and biological pretreatment require a complex maintenance process, such as uniformity, temperature control, etc. [8, 9].
In contrast, hydrothermal pretreatment is considered to be a more efficient and promising conversion process to improve digestion of lignocellulosic biomass in whole slurry. Although higher temperature (180 – 210 ℃) has been widely applied for facilitating biofuels production, especially bioethanol, it is not suitable for biogas fermentation, and relatively lower temperature (< 120 ℃) is considered to be optimal [10]. In that sense, several potential advantages could be provided, such as low energy input, low pressure requirement, avoidance of chemicals, etc. [11, 12]. Furthermore, it has been reported that the solubility of lignin could possibly be improved at temperatures higher than 90 ℃, dissolving it into soluble fractions as well as loosening the recalcitrant structure [13]. Meanwhile, efficient mixing of substrate allows uniform distribution of feedstock as the well mass transfer of hot water or stream throughout the lower temperature hydrothermal pretreatment.
Nevertheless, most of the recent studies on pretreatment of lignocellulosic biomass at high temperatures have employed temperatures ranging from 150 ℃ to 240 ℃. For instance, Ferreira et al. treated wheat straw at 200 ℃ for 5 min and 220 ℃ for 1 min to achieve 27% and 20% higher methane yields, when compared with that obtained using raw straw, respectively [14]. Furthermore, Zhou et al. demonstrated 49.7% increase in biogas yield following pretreatment at 200 ℃ [15]. Whereas, instead of using the whole slurry, these investigations had employed solid–liquid separation and utilized only the obtained insoluble fraction for biogas production, which could inevitably reduce the biogas yield because the derived soluble fraction could not be digested together, resulting in media acidification, etc. [16]. With insoluble fraction as feedstock, the biofuel yield of each unit could be improved because certain quantities of lignin and hemicellulose are removed. Nevertheless, the whole slurry should be digested in an optimal process for effective utilization of straw and higher energy recovery.
Ideally, once a part of lignin could be dissolved in water at a lower temperature, more cellulose and hemicellulose may possibly become available for degradation by anaerobic bacteria, resulting in an increase in net energy production [7]. Cao et al. reported that the lignin removal rate reached 91.02% when sweet sorghum bagasse was pretreated at 121 ℃ for 60 min [17]. Jin et al. found that alkaline pretreatment of switch grass at 100 ℃ sharply reduced the concentration required for adequate lignocellulosic degradation [18]. However, studies on optimal pretreatment temperature for RS anaerobic digestion with relatively lower temperature hydrothermal pretreatment are scarce.
Therefore, the aim of this study was to investigate the biodegradability and anaerobic digestion of RS at lower temperature (90 – 130 ℃) by using an experimental approach. Based on the results, the feasibility of practical operation of the developed approach was evaluated with whole slurry by net energy production analysis. Materials and methods will be presented in the next section, followed by the study on the pretreatment effect of whole slurry digestion, biogas production and net energy production.
RS was collected from a farm in Deyang city, China, and stored in nylon bags after natural drying. At the beginning of pretreatment, the RS was chopped into pieces of 30–50 mm in length. The inoculum for anaerobic digestion was obtained from a mesophilic anaerobic digester near Chengdu city, China, employing crop straw as feedstock. The inoculum was pre-incubated for 7 days (35.0 ±1.0 ℃) to minimize its residual biodegradable organic material content and activate the microorganisms. The main characteristics of the RS and inoculum are listed in Table S1 (Supporting information).
The hydrothermal pretreatment was performed in a 1-L stainless steel reactor as shown in Fig. S1 (Supporting information). The electric wire twined outside the reactor was used as the heat source, and internal liquid temperature was raised by heat transfer of the wall. The temperature was monitored using a temperature probe installed in the middle of the reactor. The recorded data were automatically analyzed to precisely control the heater operation. Once the liquid temperature reached the desired value, it was held at that temperature, and the heating time was recorded. As the setting time was reached, the heating process was immediately stopped and the temperature was decreased by washing with a large amount of tap water.
The RS was pretreated at temperatures of 90 ℃, 100 ℃, 110 ℃, 120 ℃, and 130 ℃ for 150 min. The initial RS to pretreated water mass ratio was 1:2. Table S2 (Supporting information) shows the RS pretreatments employed in this study.
The RS anaerobic digestion performance was evaluated by batch tests. As substrate, both the liquid and solid fractions after hydrothermal pretreatment were collected together and inoculated into 1-L glass bottle digesters with 800-mL working volume at a substrate–inoculum mass ratio of 0.5 g VS: 1 g VS. In the control, pretreatment with water under the same ambient temperature conditions, and for self-heating of aerobic fermentation, a maximum temperature of 40 ℃ was employed during the retting period. All the batch digesters were run in triplicate and under mesophilic conditions in a water bath (35 ± 0.2 ℃). The assays were terminated when the daily biogas production reached below 5% of the maximum biogas produced. To prevent scum from obstructing the biogas pipe, a stainless steel mesh was installed at the biogas outlet. The biogas produced was measured periodically through water volume displacement [19]. The statistical significance of each parameter was evaluated using modified ANOVA to account for the lack of independence of observations on a given digester, and each digester was used under only one condition.
The total solids (TS) and volatile solids (VS) contents were determined according to the Standard Methods for Examination of Water and Waste Water [20], and the volatile fatty acid (VFA) concentration was measured based on the methods of STM 5560 C [21]. Individual VFA concentrations were quantified through direct injection into a gas chromatograph with a flame ionization detector (GC-FID) [22]. Cellulose, hemicellulose, and lignin contents were measured according to the NREL LAP protocol [15]. Both cellulose and hemicellulose of RS were degraded to mono-sugars, which were quantified by HPLC with 72% and 4% sulfuric acid, respectively.
Energy production can be determined by several methods, among which net energy production could be considered as the most external indicator to reflect the energy benefits. The heat used for hydrothermal treatment could be defined as shown in Eq. S1 and Eq. S2 (Supporting information) [23].
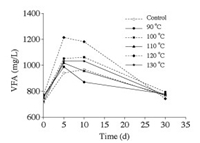
The VFA concentration is an indicator of metabolism, which is normally used to evaluate the balance between hydrolysis and methanogens [24, 25]. The VFAs concentrations of the samples are shown in Fig. 1. The VFAs concentrations reached a much higher level during the initial period, especially, the maximum VFAs concentrations at 90 ℃, 100 ℃, 110 ℃, 120 ℃, and 130 ℃ pretreatments were 2.1%, 9.7%, 5.1%, 25.5%, and 6.8% higher than that in the control, respectively, because the hydrolysis and acidification rates were accelerated by hydrothermal pretreatment [26].
|
Download:
|
| Fig. 1. VFAs production during anaerobic digestion with different hydrothermal temperatures. | |
It must be noted that when the VFAs level increased or remained at a higher level, the growth and metabolism of methanogens did not correspond to those of acid formation bacteria. As a result, methanogenesis was inhibited and biogas production ceased. As the common knowledge, as VFAs concentrations are below 1700 mg/L, it is conducive for methanogens growth and biogas production, however, the indicative VFA level could not be clearly specified because the operating conditions differed, suggesting that the relative change in the VFA concentration could also be used as an indicator [27].
Overall, pretreatment at 120 ℃ resulted in highest VFAs production, followed by pretreatments at 100 ℃ and 130 ℃. In other words, peak biogas production might have been possibly postponed, and methanogenesis might have been slightly inhibited by the accumulated VFAs. Although the variation in VFAs concentration initially appeared similar following all the pretreatments, it did not show a linear relation with increasing pretreatment temperature. On day 5, VFAs production following pretreatments at 90 ℃, 110 ℃, 120 ℃, and 130 ℃ reached the maximum, and then gradually decreased. Although pretreatments at 100 ℃ reached the maximum on day 10, its value is almost equal to that on day 5. In contrast, the control test reaches that at day 10 gradually, without quickly reaching the maximum. Furthermore, at 90 ℃, 100 ℃, 110 ℃, 120 ℃, and 130 ℃ pretreatments were initially 7.3%, 4.8%, 4.8%, 8.3%, and 0.7% higher than that noted in the control, respectively, indicating that hydrothermal pretreatment could promote hydrolysis and acidification, effectively improving the hydrolysis rate.
Lignin, which is difficult to degrade and convert to biogas through normal anaerobic digestion and hydrothermal pretreatment, is known as the undigestible part (UD), whereas cellulose and hemicellulose are defined as the digestible part (D) [15]. The lignocellulosic composition after anaerobic digestion is shown in Table 1. It can be observed from the table that the contents of cellulose, hemicellulose, and lignin were not significantly altered, because hemicellulose and cellulose exhibited obvious solubilization at approximately 150 ℃ and 160 ℃, respectively, while most of lignin was also not solubilized at this pretreatment temperature range [28, 29]. However, some previous studies have indicated that lower temperature pretreatment could possibly break lignocellulosic bonds and accelerate the hydrolysis and anaerobic digestion rates [30]. As lignin is very difficult to digest, the rates of D and UD (D:UD) deviation could be used to identify the degradation extent [31].
|
|
Table 1 Lignocellulosic composition after anaerobic digestion. |
In general, the lignin contents increased, whereas the cellulose and hemicellulose contents decreased after anaerobic digestion of hydrothermal-pretreated RS, when compared with the control. Although the D:UD rate for 130 ℃ pretreatment was slightly higher than that for the control, the lignin content was still lower, because solubilization of lignin increased with increasing hydrothermal pretreatment temperature. Furthermore, the variation trend of D: UD rate appeared to be similar to that of VFAs concentration with the increasing pretreatment temperature, with 120 ℃ pretreatment presenting the highest variation and the control (retting RS) showing the lowest variation. Moreover, the D:UD rates for 100 ℃ and 130 ℃ pretreatments were similar, and were close to that noted for the control. These findings indicated that the degradation rate of RS did not present a linear relationship with the increase in pretreatment temperature. Besides, the lignocellulosic structure could be more efficiently degraded following pretreatments at 100 ℃ and 130 ℃.
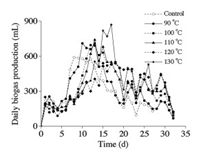
The daily biogas productions noted in all the treatment groups are shown in Fig. 2. A rapid increase in biogas production was achieved on the first day in all the treatment groups, and the peaks were reached on days 8, 10, 12, 13, 15, and 17 in control, 90 ℃, 100 ℃, 110 ℃, 120 ℃, and 130 ℃ pretreatments, respectively. The fermentation time for maximum daily biogas production increased with the increasing pretreatment temperature, which may be owing to the acceleration of degradation. This indicated that thermal pretreatment is a very effective method to enhance the hydrolysis of lignocellulose, and higher thermal temperature could postpone biogas production. Subsequently, the daily biogas productions gradually decreased, remaining at a low level until the end of the experiment. In general, no obviously inhibition phenomenon was observed during the entire anaerobic digestion.
|
Download:
|
| Fig. 2. Daily biogas production during anaerobic digestion with different hydrothermal temperatures. | |
Fig. S2 (Supporting information) illustrates the methane content in the biogas produced. The methane contents increased gradually and remained stable, but 100 ℃ pretreatment exhibited the most stable methane production during the whole anaerobic digestion, with the methane content increasing dramatically and reaching 50% on day 9, and then remaining at a stable level until the end of digestion [32]. Besides, maximum average methane content was also achieved with 100 ℃ pretreatment. These findings indicated that metabolism of anaerobic bacterias can be in a well state, and well coherent with that of acidogenesis.
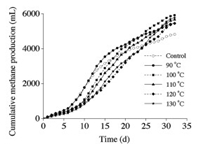
The cumulative methane production achieved with different hydrothermal pretreatment temperatures is illustrated in Fig. 3. The methane production process could be roughly divided into three stages. During the first stage (from the start to day 5), the variation trend in the control and hydrothermal pretreatment groups was similar, representing steady methanogenesis. During the second stage (days 6–25), the methane production rate presented an opposite trend with respect to VFAs concentration (Fig. 1). Following 120 ℃ pretreatment, the highest cumulative VFAs concentration and lowest methane production rate were achieved, whereas 100 ℃ pretreatment, the methane production rate increased rapidly and continuously. In the control and 90 ℃ pretreatment, the methane production rates rapidly increased in the initial half stage, but were relatively slow in the latter half stage, whereas in 110 ℃, 120 ℃, and 130 ℃ pretreatment groups, the methane production rates were relatively steady. During the third stage (days 26–32), 100 ℃ pretreatment maintained the highest cumulative methane production, and ultimately achieved the highest methane yield of 127.6 L/kg TS, which was 22.8% higher than that noted in the control (103.9 L/kg TS). The methane production could be considered as normal anaerobic digestion result, because significant variation in the methane yield was noted, ranging from 92 L/kg VS to 280 L/kg VS, depending on the straw digestion conditions [2].
|
Download:
|
| Fig. 3. Cumulative methane production during anaerobic digestion with different hydrothermal temperatures. | |
These results indicated that high degradation rate could be accomplished with suitable pretreatment temperature, rather than high pretreatment temperature, and the rate-limiting step of biogas production varied depending on the extent of lignocellulose hydrolysis. Furthermore, 100 ℃ pretreatment could achieve suitable metabolic state in an anaerobic degradation system, which improved biogas production without inhibition by the accumulated VFAs.
Thus, low temperature hydrothermal pretreatment could be considered as an effective method to improve biogas production, although further studies are needed to determine the detailed degradation mechanism.
The results of the present study demonstrated that 100 ℃ could be an ideal temperature for RS hydrothermal pretreatment to achieve desired biogas production. However, the net energy production must be evaluated to identify the feasibility of the pretreatment for practical operation. Accordingly, for the calculation of net energy production, the density of whole slurry (mixture of suspension) was considered to be 1.0 kg/L, annual average atmospheric temperature was assumed as 20 ℃, and heat capacity (equivalent to that of water) was presumed to be 4.18 J g-1 ℃-1. As the calculation did not include heat loss during preheating, inefficient heating, or waste-heat utilization, it was deemed as an ideal-case scenario.
Besides, retting pretreatment is considered to be aerobic composting without extra heat supply, whereas for hydrothermal pretreatment, temperature should be increased from 20 ℃ to 100 ℃. For high-temperature hydrothermal pretreatment comparison, 180 ℃ pretreatment was employed, which produced only 3% higher biogas yield, when compared with non-pretreated RS [10]. Table S3 (Supporting information) shows the energy requirements for retting and hydrothermal pretreatments, suggesting that very high temperature is not suitable for commercial operation. With 1.694 m3 water required to treat each unit ton of RS, 100 ℃ pretreatment could achieve 12.8% higher net biogas production from RS, when compared with the control. However, the surplus biogas could only meet 75.9% of energy requirement for increasing the water temperature. Thus, retting pretreatment might be more favorable owing to the small energy gap.
However, lower temperature hydrothermal pretreatment might be a feasible option under certain working conditions as follows: 1) mesophilic or thermophilic fermentation can be conducted with preheated feedstock (whole slurry) to maintain digestion at a stable state. Accordingly, retting RS should be heated from 20 ℃ to at least 35 ℃ (mesophilic), to achieve higher net energy production than that of retting pretreatment. 2) RS can be heated with less pretreated water. 3) Excess heat captured from other processes, such as solar energy or heat from combination heat and power system, could be used to heat pretreated water (> 40 ℃). 4) Some waste heat could be reused for reducing biogas demand.
In conclusion, the RS anaerobic digestion performance substantially increased with thermal pretreatment at relatively low temperature (≤ 100 ℃), which was beneficial for improving lignocellulosic degradation, reducing fermentation period, and enhancing methane yield. However, as the surplus biogas failed to meet the whole slurry heating requirement, the net energy production appeared to be lower than that noted in the control. Nevertheless, this method could still be a feasible approach for mesophilic or thermophilic fermentation. Besides, better net energy production could be achieved in practical applications with an optimal design of the integral anaerobic digestion system or extra accessible heat source. Future research should focus on the degradation mechanism of lower temperature hydrothermal pretreatment as well as optimization of the pretreatment duration and procedure.
AcknowledgmentsThe authors gratefully acknowledge financial support from Special Fund for Agro-scientific Research in the Public Interest (No. 201403019) and Integrated Rural Energy Development Project of MOA (No. J576) and the Sichuan Province Science and Technology Support Project (No. 18RKX0185) and the International Clean Energy Talent Program of China Scholarship Council (No. 201802180094) and the Agricultural Science and Technology Innovation Program (ASTIP) of the Chinese Academy of Agricultural Sciences.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.03.018.
| [1] |
H. Li, Y. Cao, X. Wang, et al., Bioenerg. Res. 10 (2017) 949-957. DOI:10.1007/s12155-017-9845-4 |
| [2] |
W. Mussoline, G. Esposito, A. Giordano, P. Lens, Crit. Rev. Environ. Sci. Technol. 43 (2013) 895-915. DOI:10.1080/10643389.2011.627018 |
| [3] |
M.P. Taylor, K.L. Eley, S. Martin, M.I, et al., Trends Biotechnol. 27 (2009) 398-405. DOI:10.1016/j.tibtech.2009.03.006 |
| [4] |
G. Brodeur, E. Yau, K. Badal, J, et al., Enzyme Res. 23 (2011) 2937-2968. |
| [5] |
J. Ariunbaatar, A. Panico, G. Esposito, F. Pirozzi, P.N.L. Lens, Appl. Energy 123 (2014) 143-156. DOI:10.1016/j.apenergy.2014.02.035 |
| [6] |
H. Palonen, F. Tjerneld, G. Zacchi, M. Tenkanen, J. Biotechnol. 107 (2004) 65-72. DOI:10.1016/j.jbiotec.2003.09.011 |
| [7] |
M.J. Taherzadeh, K. Karimi, Int. J. Mol. Sci. 9 (2008) 1621-1651. DOI:10.3390/ijms9091621 |
| [8] |
L. He, H. Huang, Z. Lei, C. Liu, Z. Zhang, Bioresour. Technol. 171 (2014) 145-151. DOI:10.1016/j.biortech.2014.08.049 |
| [9] |
L.M. López González, R.I. Pereda, J. Dewulf, et al., Bioresour. Technol. 169 (2014) 284-290. DOI:10.1016/j.biortech.2014.06.107 |
| [10] |
D. Wang, F. Shen, G. Yang, et al., Bioresour. Technol. 249 (2017) 117-124. |
| [11] |
F. Hu, A. Ragauskas, Bioenerg. Res. 5 (2012) 1043-1066. DOI:10.1007/s12155-012-9208-0 |
| [12] |
Z. Merali, S.R.A. Collins, A. Elliston, et al., Biotechnol. Biofuels 131 (2015) 226-234. |
| [13] |
S.V. Vassilev, D. Baxter, C.G. Vassileva, Fuel 112 (2013) 391-449. DOI:10.1016/j.fuel.2013.05.043 |
| [14] |
L.C. Ferreira, A. Donoso-Bravo, P.J. Nilsen, F. Fdz-Polanco, S.I. Pérez-Elvira, Bioresour. Technol. 143 (2013) 251-257. DOI:10.1016/j.biortech.2013.05.065 |
| [15] |
X. Zhou, Q. Li, Y. Zhang, Y. Gu, Bioresour. Technol. 224 (2016) 721-726. |
| [16] |
J.C. Motte, R. Escudie, J. Hamelin, et al., Bioresour. Technol. 173 (2014) 185-192. DOI:10.1016/j.biortech.2014.09.015 |
| [17] |
W. Cao, S. Chen, R. Liu, R. Yin, X. Wu, Bioresour. Technol. 111 (2012) 215-221. DOI:10.1016/j.biortech.2012.02.034 |
| [18] |
G. Jin, T. Bierma, P.M. Walker, J. Environ. Sci. Health A. Environ. Sci. Eng. 49 (2014) 565-574. DOI:10.1080/10934529.2014.859453 |
| [19] |
P.L. Thompson, F.W. Jiencke, S.W. Reinhart, M.E. Reha, S.S. Byrne, Water Environ. Res. 85 (2013) 327-330. DOI:10.2175/106143012X13415215907176 |
| [20] |
American Public Health Association (APHA), Standard Methods for the Examination of Water and Wastewater, Washington, DC, (2005).
|
| [21] |
M.A. Khan, H.H. Ngo, W.S. Guo, et al., Bioresour. Technol. 219 (2016) 738-748. DOI:10.1016/j.biortech.2016.08.073 |
| [22] |
M.A. Ullah, K.H. Kim, J.E. Szulejko, J. Cho, Anal. Chim. Acta 820 (2014) 159-167. DOI:10.1016/j.aca.2014.02.012 |
| [23] |
Y. Liu, Y. Chen, T. Li, D. Wang, D. Wang, Biosyst. Eng. 163 (2017) 116-133. DOI:10.1016/j.biosystemseng.2017.09.002 |
| [24] |
N. Murali, S. Fernandez, B.K. Ahring, Bioresour. Technol. 227 (2017) 197-204. DOI:10.1016/j.biortech.2016.12.012 |
| [25] |
G.W. Park, I. Kim, K. Jung, et al., Bioprocess Biosyst. Eng. 38 (2015) 1623-1627. DOI:10.1007/s00449-015-1387-6 |
| [26] |
A. Valentini, G. Garuti, A. Rozzi, A. Tilche, Water Sci. Technol. 36 (1997) 239-246. |
| [27] |
J. Shi, Z.J. Wang, J.A. Stiverson, Z.T. Yu, Y.B. Li, Bioresour. Technol. 136 (2013) 574-581. DOI:10.1016/j.biortech.2013.02.073 |
| [28] |
G. Yu, S. Yano, H. Inoue, et al., Appl. Biochem. Biotechnol. 160 (2010) 539-551. DOI:10.1007/s12010-008-8420-z |
| [29] |
G. Garrote, H. Domínguez, J.C. Parajó, Holz. Roh.-Werkst. 57 (1999) 191-202. DOI:10.1007/s001070050039 |
| [30] |
Q. Wang, C. Noguchi, Y. Hara, et al., Bioresour. Technol. 18 (1997) 999-1008. |
| [31] |
L. Wanwu, K. Habiba, Z. Zhe, et al., Appl. Energy 226 (2018) 1219-1228. DOI:10.1016/j.apenergy.2018.05.055 |
| [32] |
F. Yu, X. Xu, Appl. Energy 134 (2014) 102-113. DOI:10.1016/j.apenergy.2014.07.104 |
 2019, Vol. 30
2019, Vol. 30 


