b Shanghai Collaborative Innovation Center for Biomanufacturing Technology, Shanghai 200237, China
The endoparasitic sedentary root-knot nematodes (RKNs), Meloidogyne spp., are the most damaging nematode group in the world as they can cause considerable yield losses to most cultivated plant species. It is estimated to account for greater than 50% of all nematicide use and 5% of crop loss globally, resulting in $50 billion annually [1, 2].
For decades, chemical nematicides are considered as the most effective approach in the management of nematodes, and this is unlikely to change in the near future [1]. Soil fumigants, a few commercial organophosphorous and carbamate nematicides are mainly applied for the control of RKNs. These nematicides have been restricted or withdrawn from the market because of high environmental impact caused by their repeated applications over the years [3]. At present, fosthiazate and abamectin are the most commonly used nematicides in the market although resistance development in the target pathogens are increasing [4]. And abamectin has been listed in the Department Banned Pesticide Active List UPDATE. The global nematicide market was valued at $1 billion in 2011, the market is estimated to increase to $1.4 billion by 2020 [5-7]. Therefore, despite that the newly developed trifluorobutenesulfanyl, fluensulfone and fluopyram have been released to the market, the discovery and development of new nematicides with high nematicidal activity and low toxicity to non-target organisms are essential in the defense of crops.
Piperazine derivatives have been reported to show significant biological actions such as anti-microbial [8], anti-depressant [9], anti-parkinson [10], anti-inflammatory [11], antioxidant [12], antidiabetic [13], antiproliferative [14] and anti-histaminic activity [15]. To the best of our knowledge, about 30 kinds of commercial pharmaceuticals were synthesized from piperazine. Some of the drugs include piperazine as linker (Fig. 1). Moreover, some of the commercial anthelmintic drugs for nematode parasites of livestock are piperazine derivatives, for instance, diethylcarbamazine, piperazine phosphate and piperazine citrate (Fig. S1 in Supporting information). And several piperazine derivatives were reported to have insecticidal and fungicidal activities [16-18]. Piperazine attracted our attention in the design of new nematicides.
|
Download:
|
| Fig. 1. Piperazine as linker in drugs. | |
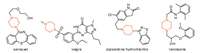
Moreover, some fused heterocyclic compounds with nematicidal activities were reported [19, 20]. According to our previous research (Fig. S2 in Supporting information), 1,2,3- benzotriazin-4-one derivatives exhibited good nematicidal activities against Meloidogyne incognita [21-23]. Summarizing the prophase structures, it was found that the structural frame was made up of active structure 1,2,3-benzotriazin-4-one, linker and fragment. Based on the research and investigation above, the structure containing 1,2,3-benzotriazin-4-one as pharmacophore, piperazine as a part of linker and some active fragments as a varying part was designed and synthesized (Fig. 2). The fragments included some heterocycles selected from pesticides, fungicides, nematicides and natural products. We conjectured that the introduction of piperazine group and diverse fragments into 1,2,3-benzotriazin-4-one scaffolds might generate novel lead molecules with better nematicidal properties. Then, we tested their activity in vivo and in vitro against M. incognita and discussed the preliminary structure and activity relationship (SAR), which would provide the basis for further research.
|
Download:
|
| Fig. 2. Design of title compounds. | |
First of all, we synthesized all the title compounds as described in Scheme 1. General procedures for preparation of intermediates and spectra data were provided in Supporting information.

|
Download:
|
| Scheme 1. Synthetic procedure for title compounds. Reagents and conditions: (a) triphosgene (BTC), THF, –5 ℃, 5 h. (b) (NH4)2CO3, 1, 4-dioxane, 60 ℃, 8 h. (c) ⅰ. NaNO2, 1.0 mol/L HCl, H2O, 0 ℃, 2 h; ⅱ. 30% NaOH, pH 8, 15 min; (d) 1, 2-dibromoethane, K2CO3, acetone, reflux 5–8 h. | |
Synthesis of 3-(2-(piperazin-1-yl) ethyl)benzo[d][1,2,3]triazin- 4(3H)-one (A1). A suspension of 3-(2-bromoethyl) benzo[d][1,2,3] triazin-4(3H)-one (35 mmol), piperazine (105 mmol), and tetrahydrofuran (200 mL) was refluxed for 5–8 h. The reaction process was monitored by TLC. After the complete consumption of 3-(2- bromoethyl)benzo[d][1,2,3]triazin-4(3H)-one, the reaction mixture was cooled to room temperature and evaporated under reduced pressure, and then water (200 mL) was added to the residue, which was extracted with DCM (100 mL × 5). The organic layer was dried over anhydrous Na2SO4 and concentrated to give compound A1 in yield of 72%.
3-(2-(Piperazin-1-yl)ethyl)benzo[d][1,2,3]triazin-4(3H)-one (A1): White solid, mp 65.6–65.8 ℃, Yield: 72%; 1H NMR (400 MHz, DMSO-d6): δ 8.26 (d, 1H, J = 8.0 Hz), 8.20 (d, 1H, J = 8.0 Hz), 8.09 (t, 1H, J = 7.6 Hz), 7.94 (t, 1H, J = 7.6 Hz), 4.50 (t, 2H, J = 6.8 Hz), 3.17 (br, s, masked with water, 1 H), 2.74 (t, 2H, J = 6.8 Hz), 2.69–2.55 (m, 4 H), 2.46–2.32 (m, 4 H); 13C NMR (100 MHz, DMSO-d6): δ 154.7, 143.6, 135.4, 132.9, 127.9, 124.5, 119.1, 56.3, 53.7, 46.2, 45.4; HRMS (ESI) calcd. for C13H18N5O [M+H]+: 260.1511, found: 260.1513.
Synthesis of 3-(2-(4-methylpiperazin-1-yl)ethyl)benzo[d] [1,2,3]triazin-4(3H)-one (A46): A suspension of 3-(2-bromoethyl)benzo[d][1,2,3]triazin-4(3H)-one (3.5 mmol), 1-methylpiperazine (4.0 mmol), potassium carbonate (4.0 mmol), and tetrahydrofuran (20 mL) was refluxed for 5–8 h. The reaction process was monitored by TLC. After the complete consumption of 3-(2-bromoethyl)benzo[d][1,2,3]triazin-4(3H)-one, the reaction mixture was cooled to room temperature and evaporated under reduced pressure, and then water (50 mL) was added to the residue, which was extracted with DCM (50 mL × 4). The organic layer was dried over anhydrous Na2SO4 and concentrated to give compounds A46 in yields of 67%.
3-(2-(4-Methylpiperazin-1-yl)ethyl)benzo[d][1,2,3]triazin-4 (3H)-one (A46): White solid, mp 120.0–121.6 ℃, Yield: 67%; 1H NMR (400 MHz, DMSO-d6): δ 8.26 (dd, 1H, J = 8.0, 1.0 Hz), 8.21 (d, 1H, J = 7.8 Hz), 8.14–8.06 (m, 1 H), 8.00–7.89 (m, 1 H), 4.51 (t, 2H, J = 6.6 Hz), 3.44–3.35 (m, 4 H), 2.77 (t, 2H, J = 6.6 Hz), 2.54–2.47 (m, 2 H), 2.33–2.25 (m, 2 H), 2.13 (s, 3 H); 13C NMR (100 MHz, DMSO-d6): δ 154.7, 143.6, 135.4, 132.9, 127.9, 124.5, 119.1, 55.6, 54.5, 52.3, 46.3, 45.5; HRMS (EI) calcd. for C14H19N5O [M]+: 273.1590, found: 273.1588.
Synthesis of A2–A54, A49, A54 (Method A): A mixture of substituted 3-(2-(piperazin-1-yl)ethyl)benzo[d][1,2,3]triazin-4 (3H)-one (3 mmol), triethylamine (4 mmol) and DCM (10 mL) was stirred at 0 ℃ for 20 min. Then, acyl chloride compound (or aryl sulfuryl chloride compound) (3.5 mmol) dissolved in DCM (10 mL) was added dropwise to the above solution for 10 min. Then the mixture was stirred at room temperature. The reaction process was monitored by TLC. After the complete consumption of 3-(2- (piperazin-1-yl)ethyl)benzo[d][1,2,3]triazin-4(3H)-one, the reaction mixture was evaporated under reduced pressure, and water (100 mL) was added to the residue, which was extracted with DCM (50 mL × 2). The organic layer was dried over anhydrous Na2SO4, concentrated, and purified with flash chromatography on silica gel, eluting with petroleum ether (60–90 ℃)/EtOAc to afford compounds in yield of 40%–87%.
3-(2-(4-Benzoylpiperazin-1-yl)ethyl)benzo[d][1,2,3]triazin-4 (3H)-one (A2): White solid, mp 137.4–139.2 ℃, Yield: 87%; 1H NMR (400 MHz, DMSO-d6): δ 8.27 (d, J = 8.0 Hz, 1 H), 8.21 (d, J = 8.0 Hz, 1 H), 8.10 (t, J = 7.6 Hz, 1 H), 7.94 (t, J = 7.5 Hz, 1 H), 7.48 – 7.40 (m, 3 H), 7.39 – 7.33 (m, 2 H), 4.54 (t, J = 6.4 Hz, 2 H), 3.65 – 3.45 (m, 2 H), 3.31 – 3.13 (m, 2 H), 2.83 (t, J = 6.4 Hz, 2 H), 2.64 – 2.36 (m, 4 H). 13C NMR (100 MHz, DMSO-d6): δ 168.8, 154.8, 143.6, 135.9, 135.4, 132.9, 129.4, 128.4, 127.9, 126.8, 124.6, 119.1, 55.4, 46.2; HRMS (EI) calcd. for C20H21N5O2 [M]+: 363.1695, found: 363.1693.
Synthesis of A47, A48, A51, A52 (Method B): A mixture of 3-(2- (piperazin-1-yl)ethyl)benzo[d][1,2,3]triazin-4(3H)-one (3 mmol), K2CO3 (4 mmol) and CH3CN (10 mL) was stirred at 0 ℃ for 20 min. Then, bromo-compound (or chloro-compound) (3.0 mmol) dissolved in CH3CN (10 mL) was added dropwise to the above solution for 10 min. Then the mixture was stirred at 50 ℃, the reaction process was monitored by TLC. After the complete consumption of 3-(2-(piperazin-1-yl)ethyl)benzo[d][1,2,3] triazin- 4(3H)-one, the reaction was cooled to room temperature. Then the reaction mixture was evaporated under reduced pressure, and water (100 mL) was added to the residue, which was extracted with DCM (50 mL × 2). The organic layer was dried over anhydrous Na2SO4, concentrated, and purified with flash chromatography on silica gel, eluting with petroleum ether (60–90 ℃)/EtOAc to afford compounds in yield of 61%–80%.
3-(2-(4-(Cyclopropylmethyl)piperazin-1-yl)ethyl)benzo[d] [1,2,3]triazin-4(3H)-one (A47):White solid, mp 107.5–108.9 ℃, Yield: 61%; 1H NMR (400 MHz, DMSO-d6): δ 8.22 (d, 1H, J = 7.8 Hz), 8.16 (d, 1H, J = 8.2 Hz), 8.05 (t, 1H, J = 7.6 Hz), 7.89 (t, 1H, J = 7.6 Hz), 4.46 (t, 2H, J = 6.4 Hz), 3.38 – 3.29 (m, 4 H), 2.73 (t, 2H, J = 6.4 Hz), 2.40–2.32 (m, 4 H), 2.10 (d, 2H, J = 5.2 Hz), 1.20–1.16 (m, 1 H), 0.38 (d, 2H, J = 7.6 Hz), -0.01 (d, 2H, J = 4.2 Hz). 13C NMR (100 MHz, DMSO-d6): δ 154.7, 143.6, 135.4, 132.9, 127.9, 124.5, 119.1, 62.6, 55.6, 52.6, 52.4, 46.3, 8.1, 3.6; HRMS (EI) calcd. for C17H23N5O [M]+: 313.1903, found: 313.1904.
Synthesis of A50, A53 (Method C): A mixture of 3-(2- (piperazin-1-yl)ethyl)benzo[d][1,2,3]triazin-4(3H)-one (3 mmol), triethylamine (4 mmol) and DCM (10 mL) was stirred at 0 ℃ for 20 min. Then, 5-isopropyl-2-methylphenyl carbonochloridate (phenyl carbonochloridate for A50) (3 mmol) dissolved in DCM (10 mL) was added dropwise to the above solution for 10 min, then the mixture was stirred at room temperature. The reaction process was monitored by TLC. After the complete consumption of 3-(2- (piperazin-1-yl)ethyl)benzo[d][1,2,3]triazin-4(3H)-one, the reaction mixture was evaporated under reduced pressure, and water (100 mL) was added to the residue, which was extracted with DCM (50 mL × 2). The organic layer was dried over anhydrous Na2SO4, concentrated, and purified with flash chromatography on silica gel, eluting with petroleum ether (60–90 ℃)/EtOAc to afford compound A53 in yield of 67% (A50 in yield of 80%).
Phenyl 4-(2-(4-oxobenzo[d][1,2,3]triazin-3(4H)-yl)ethyl)-piperazine-1-carboxylate (A50): White solid, mp 129.5–130.5 ℃, Yield: 76%; 1H NMR (400 MHz, CDCl3): δ 8.37 (dd, 1H, J = 8.0, 1.0 Hz), 8.17 (d, 1H, J = 8.0 Hz), 8.01–7.91 (m, 1 H), 7.85–7.78 (m, 1 H), 7.35 (t, 2H, J = 8.0 Hz), 7.18 (t, 1H, J = 7.4 Hz), 7.13–7.05 (m, 2 H), 4.65 (t, 2H, J = 6.4 Hz), 3.63–3.55 (m, 4 H), 2.97 (t, 2H, J = 6.4 Hz), 2.68–2.58 (m, 4 H); 13C NMR (100 MHz, CDCl3): δ 155.7, 153.6, 151.4, 144.3, 134.8, 132.4, 129.3, 128.3, 125.3, 125.1, 121.7, 119.8, 56.1, 52.7, 46.6, 44.4, 43.9; HRMS (ESI) calcd. for C20H22N5O3 [M+H]+: 380.1723, found: 380.1722.
Furthermore, nematicidal activities against M. incognita of all compounds were evaluated. The second-stage juveniles (J2) of M. incognita used in all tests were cultured by Huzhou Modern Agricultural Biotechnology Innovation Center, Chinese Academy of Sciences, China.
In vivo. All compounds (A1–A54) were dissolved with acetone (DMF was toxic to cucumber seedling in our test model), then diluted with distilled water to obtain series concentration of 20.0 mg/L for bioassays. The final concentration of acetone in each treatment never exceeded 1% (v/v). The one-week age cucumber seedlings were replanted in sterilized sand in test tubes (one seedling per test tube, tube size: 20 mm × 250 mm), and the roots of each seedling were treated with 3 mL of test solution. Then approximately 2000 living J2 nematodes were inoculated into the rhizosphere sand of each host plant. Fenamiphos and avermectin (B1) at concentration of 20.0 mg/L served as positive control, and the negative control group was prepared in the same way but lacked the tested compound. Distilled water without nematodes served as blank control. Each treatment was replicated four times and the experiment was repeated three times. All the above test tubes were incubated at 20–25 ℃ for 45 days, for 10 h in the daylight and 14 h in the dark per day. The number of root knots in each test tube was counted and recorded a score. Scoring criteria: 0, 0–5 knots; 5, 6–10 knots; 10, 11–20 knots; 20, more than 20 knots. The inhibition activity on J2 of M. incognita was calculated by comparison with the negative control group (Eq. 1):
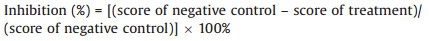
|
(1) |
In vitro. Pure compounds (A1–A54) were dissolved in DMF and diluted with distilled water to obtain stock solutions of double the treatment concentration. Then, 2 mL of J2 aqueous suspension containing approximately 200 living nematodes was added to a 6 cm diameter Petri dish and treated with 2 mL of the above solution, meanwhile providing series concentrations of 50.0, 25.0, 10.0, 5.0, and 1.0 mg/L. The final concentration of DMF in each treatment never exceeded 1% (v/v). Avermectin (B1) at the above same treatment concentrations served as a positive control, and the negative control group was prepared in the same way but lacked the tested compound. Distilled water served as a blank control. All of the above test dishes were covered with the laboratory parafilm to avoid the possible evaporation or pollution. Each treatment was incubated at 25 ℃ for 24 h and had three repetitions. Nematodes in each test dish were collected after washing in sterile water through a 500-mesh sieve, and finally, the activities of tested compounds were monitored under a microscope by recording the death rates of tested nematodes. Nematodes that did not move when prodded with a needle were considered dead. The LC50 values of tested compounds were calculated using the probit method.
The in vivo nematicidal activities of title compounds (A1–A54) against M. incognita at the concentration of 20 mg/L were listed in Table 1.
|
|
Table 1 Inhibition rate of compounds A1–A54 against M. incognita in test tubes at the concentration of 20 mg/L. |
As shown in Table 1, the preliminary bioassays exhibited that some of the target compounds had good inhibitory activity, such as compound A6, A8, A21, A28 and A38 exhibited more than 50% inhibition. Especially compound A6 displayed 71.4% inhibition against M. incognita at the concentration of 20 mg/L.
Since compound A1 as an intermediate displayed 10% inhibition, A2 was synthesized with 28.6% inhibition against M. incognita at the concentration of 20 mg/L. Based on the structure of A2, different substituents were introduced into phenyl ring and 1,2,3-benzotriazin-4-one, then a series of compounds were synthesized to analyze the SAR. When electron-donating groups CH3, OCH3, electron-withdrawing groups OCF3, NO2 or halogen group Cl, were introduced into the phenyl ring, compounds A5, A6, A8, A10 showed higher inhibitory activity than others, which indicated that the activities varied significantly depending on the types and positions of the substituents. Based on the analysis of the SAR of the substituents on phenyl ring, NO2 was fixed at position 2 on phenyl ring and then different types and positions of the substituents on 1,2,3-benzotriazin-4-one was investigated further. When electron-donating group OCH3, electron-withdrawing group NO2 or halogen group Cl were introduced into different position of 1,2,3-benzotriazin-4-one, all the substituted compounds exhibited lower inhibitory activity than compound A6 and A8, which had no substituents on 1,2,3- benzotriazin-4-one. Only A21, A28 kept more than 50% activity at the concentration of 20 mg/L. The importance of fluorine in medicinal chemistry is well recognized [24]. Fluorine atom also was introduced in this scaffold. Compound A29 with fluorine at para-position of phenyl ring and A30 with fluorine at the 2, 6- position of phenyl were synthesized. Unfortunately, these two compounds had no inhibition activity against M. incognita at the concentration of 20 mg/L. Since compound A6 bearing NO2 at 2- position had the best inhibitory activity among the substituted benzene compounds, compound A31 bearing CN and A32 bearing CF3 at 2-position of phenyl ring were synthesized. But the inhibitory activity of A31 and A32 were lower than A6 at the concentration of 20 mg/L. As A10 bearing Cl at 3-position and A11 bearing Cl at 4-position of phenyl ring showed certain inhibitory activity, dichloro-substituted compound A33 at the 3- and 4- position was synthesized, but A33 showed lower inhibition activity than A10 against M. incognita at the concentration of 20 mg/L. Similarly, compound A34 bearing NO2 at 2- and 4- position exhibited lower inhibition activity than A28 bearing NO2 at 2-position and A30 bearing NO2 at 4-position.
In order to increase the diversity of the structure, some heterocycles selected from pesticides, fungicides, nematicides and natural products were also introduced. Among the synthesized compounds (A35–A45), only A38 bearing fragment from benzothiadiazole showed higher activity than 40% at the concentration of 20 mg/L and were worth further study. In addition, compounds A49, A50 bearing benzyl group, phenoxy were synthesized. The activities were 10.5% and 36.0%, respectively. At last, in order to investigate the effect of carbonyl in the fragment, we removed the carbonyl group and synthesized A46 bearing methyl, A47 bearing cyclopropyl methylene, A48 and A54 bearing benzyl group (fragment from fluazaindolizine). It was found that the inhibition activity was disappointing, which further confirmed the importance of carbonyl in the structure.
In addition, all of the synthesized compounds had no inhibitory activity at the concentration of 20 mg/L in vitro nematicidal evaluation (LC50 values of tested compounds were not displayed). The significant difference between in vivo and in vitro attracted our attention. These compounds may have different mechanism of action compared with commercial nematicides. Further research is still needed to verify the possible mechanism.
In conclusion, a series of novel 3-(2-(piperazin-1-yl)ethyl) benzo[d][1,2,3]triazine-4(3H)-one derivatives containing different modified groups using piperazine as linker were designed and synthesized. Some of them exhibited good in vivo inhibitory activities at 20.0 mg/L, which implied that the structure scaffold of 3-(2-(piperazin-1-yl)ethyl)benzo[d][1,2,3]triazin-4(3H)-one derivative containing piperazine and different modified group is a potential active structure to be worth studying further.
AcknowledgmentsThis work was financial supported by the National Natural Science Foundation of China (No. 21672061), National Key Research Program of China (Nos. 2017YFD0200505 and 2018YFD0200105). This work was also supported by the Fundamental Research Funds for the Central Universities (No. 222201718004). We are very thankful to Dr. Bingli Gao and his co-workers in Huzhou Modern Agricultural Biotechnology Innovation Center, Chinese Academy of Sciences, China, for the nematicidal evaluation.
Appendix A. Supplementary dataSupplementary material related to this article can be found, inthe online version, at doi:https://doi.org/10.1016/j.cclet.2019.02.033.
| [1] |
P.P.J. Haydock, S.R. Woods, I.G. Grove, et al., Chemical Control of Nematodes. Wallingford: CABI Publishing, 2006: pp. 392-410.
|
| [2] |
P. Abad, J. Gouzy, J.M. Aury, et al., Nat. Biotechnol. 26 (2008) 909-915. DOI:10.1038/nbt.1482 |
| [3] |
M.D. Whorton, D.E. Foliart, Res. Mutat., Rev. Genet. Toxicol. 123 (1983) 13-30. DOI:10.1016/0165-1110(83)90044-1 |
| [4] |
H.C. Meher, V.T. Gajbhiye, G. Chawla, et al., Pest Manage. Sci. 65 (2009) 1201-1207. DOI:10.1002/ps.v65:11 |
| [5] |
G.P. Lahm, J. Desaeger, B.K. Smith, et al., Bioorg. Med. Chem. Lett. 27 (2017) 1572-1575. DOI:10.1016/j.bmcl.2017.02.029 |
| [6] |
T.R. Faske, K. Hurd, J. Nematol. 47 (2015) 316-321. |
| [7] |
Y. Oka, S. Shuker, N. Tkachi, Pest Manage. Sci. 68 (2012) 268-275. DOI:10.1002/ps.v68.2 |
| [8] |
L.L. Gan, B. Fang, C. He, Bull. Korean Chem. Soc. 31 (2010) 3684-3692. DOI:10.5012/bkcs.2010.31.12.3684 |
| [9] |
J. Kossakowski, M. Krawiecka, B. Kuran, Molecules 11 (2006) 615-626. DOI:10.3390/11080615 |
| [10] |
J.B. Acri, B.K. Siedleck, J.M. Witkin, J. Pharmacol. Exp. Ther. 277 (1996) 198-206. |
| [11] |
J.C. Lien, L.J. Huang, J.P. Wang, et al., Bioorg. Med. Chem. 5 (1997) 2111-2120. DOI:10.1016/S0968-0896(97)00133-8 |
| [12] |
M. Kimura, T. Masuda, K. Yamada, et al., Bioorg. Med. Chem. Lett. 14 (2004) 4287-4290. DOI:10.1016/j.bmcl.2004.05.091 |
| [13] |
G. Le Bihan, F. Rondu, A. Pele-Tounian, et al., J. Med. Chem. 42 (1999) 1587-1603. DOI:10.1021/jm981099b |
| [14] |
C.S. Ananda Kumar, S.B. Benaka Prasad, K. Vinaya, et al., Eur. J. Med. Chem. 44 (2009) 1223-1229. DOI:10.1016/j.ejmech.2008.09.025 |
| [15] |
B.C. Sasse, U.R. Mach, J. Leppaenen, et al., Bioorg.Med.Chem. 15 (2007) 7258-7273. DOI:10.1016/j.bmc.2007.08.034 |
| [16] |
Y. Zhang, Y.Z. Zhan, Y. Ma, et al., Chin. Chem. Lett. 29 (2018) 441-446. DOI:10.1016/j.cclet.2017.08.035 |
| [17] |
L.Y. Zhang, B.L. Wang, Y.Z. Zhan, et al., Chin. Chem. Lett. 27 (2016) 163-167. DOI:10.1016/j.cclet.2015.09.015 |
| [18] |
B.L. Wang, L.Y. Zhang, X.H. Liu, et al., Bioorg. Med. Chem. Lett. 27 (2017) 5457-5462. DOI:10.1016/j.bmcl.2017.10.065 |
| [19] |
H. Sun, H. Li, J. Wang, et al., Chin. Chem. Lett. 29 (2018) 977-980. DOI:10.1016/j.cclet.2017.10.015 |
| [20] |
L. Pan, X.Z. Li, D.A. Sun, et al., Chin. Chem. Lett. 27 (2016) 375-379. DOI:10.1016/j.cclet.2016.01.029 |
| [21] |
G.L. Wang, X.L. Chen, Y.Y. Deng, et al., J. Agric. Food Chem. 63 (2015) 6883-6889. DOI:10.1021/acs.jafc.5b01762 |
| [22] |
G.L. Wang, X. Chen, Y.N. Chang, et al., Chin. Chem. Lett. 26 (2015) 1502-1506. DOI:10.1016/j.cclet.2015.10.024 |
| [23] |
Y.N. Chang, J.W. Zhang, X.L. Chen, et al., Bioorg. Med. Chem. Lett. 27 (2017) 2641-2644. DOI:10.1016/j.bmcl.2016.12.065 |
| [24] |
K.L. Kirk, J. Fluorine Chem. 127 (2006) 1013-1029. DOI:10.1016/j.jfluchem.2006.06.007 |
 2019, Vol. 30
2019, Vol. 30