b Collaborative Innovation Center of Chemical Science and Engineering(Tianjin), Tianjin 300072, China
Macrocyclic compounds are a series of cyclic oligomers containing repeating units including crown ethers, cyclodextrins, calixarenes, pillararenes and cucurbiturils. Also known as the host molecules, these macrocyclic compounds could bind various organic/inorganic/biological guest molecules into their cavities to form host-guest complexes. The host-guest interaction, a synergistic effect of several non-covalent interactions including van der Waals, hydrophobic, hydrogen bonding, and electrostatic interactions, is the basis of molecular recognition and sensing [1-9]. Meanwhile, the pre-organized structure of macrocyclic compounds also inspired the supramolecular assembly which is another research hotspot of supramolecular chemistry. Many successful studies on macrocyclic supramolecular systems were reported, such as catenane, rotaxane, molecular switch, fluorescent chemosensor, fluorescent gel, and so on [10-15]. However, the poor water solubility of many macrocycles greatly limits the development of supramolecular chemistry in biological science because aqueous environment is where most biological processes happened. Based on this reason, many endeavors are contributed to the water-soluble macrocyclic supramolecular systems.
Calixarenes are known as the third generation of macrocyclic molecules. Since Shinkai et al. reported a water-soluble calixarene derivative named p-sulfonatocalix[n]arenes (SCnAs, n = 4-8), various biomedical and biological applications based on calixarenes have been widely developed [16]. SCnAs have many advantageous features as (1) SCnAs can be easily prepared with the direct sulfonation of calixarenes; (2) the cavities of SCnAs can strongly bind guests through hydrophobic and π-stacking interactions in aqueous media; (3) the negative-charged sulfonate groups provide anchoring points towards positively charged guest molecules through electrostatic interaction [17-19]; (4) the good watersolubility and biocompatibility of SCnAs are indispensable in biological and biomedical field. Amphiphilic SCnA which contains a hydrophilic macrocyclic skeleton and several hydrophobic alkyl chains is another kind of widely developed calixarene. Several characters of these amphiphilic SCnAs are as follow: (1) the amphiphilic structure bring the self-assembly ability; (2) the hydrophobic alkyl chains provide strong hydrophobic interactions for the formation of amphiphilic assembly; (3) the modification of large steric hindrance group at the lower rim make the cavity more rigid which enhances the binding ability towards planar cationic guests and decreases the binding ability towards spherical cationic guests.
This review mainly summarizes our recent researches on the supramolecular selective binding and molecular assembly based on calixarene/pillararene. Several representative examples were provided (Scheme 1) to expound the progress in the area of recognition and sensing, multi-functional assembly and crosslinked multi-dimensional materials.
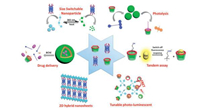
|
Download:
|
| Scheme 1. Calixarene/pillararene-based supramolecular selective binding and molecular assembly. | |
2. Recognition and sensing
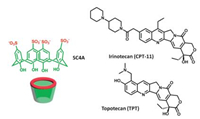
We previously reported the binding behaviors of sulfonatocalix [4]arene (SC4A) with topotecan (TPT), a chemotherapy agent used clinically in the treatment of ovarian, small cell lung and cervical cancer (Fig. 1) [20]. The stronger interaction between the quinoline ring as well as the dimethylaminomethyl group of TPT and SC4A aromatic ring was confirmed by UV/vis spectroscopy, DSC, and 2D NMR. More importantly, the water-solubility of TPT was increased by the complexation of SC4A, which made the complex a novel formulation of TPT for medicine. In addition to TPT, the interaction between irinotecan (CPT-11), a chemotherapy agent used clinically to tread advanced colorectal cancer, lung cancer and malignant lymphoma, and SC4A was also investigated [21]. Same as the case of CPT, SC4A also solubilized CPT-11 to high levels. Moreover, SC4A can increase the stability of hydroxyl lactone ring of CPT-11 and increase the antiproliferative activity.
|
Download:
|
| Fig. 1. The structures of sulfonatocalix[4]arene, irinotecan and topotecan. | |
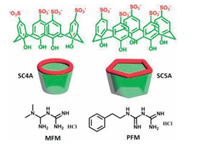
The binding behaviors of sulfonatocalix[4]arene (SC4A) and sulfonatocalix[5]arene (SC5A) with metformin (MFM) and phenformin (PFM), two important biguanidiniums molecules for treatment of hyperglycemia in patients with noninsulin-dependent diabetes mellitus, were also studied to understand the inclusion phenomena, recognition mechanism, and thermodynamic origins of SCnAs more systematically and comprehensively (Fig. 2) [22]. Both the alkyl and aromatic portions of MFM and PFM are inserted into the hydrophobic cavity of calixarene and the biguanidinium protion is captured by the sulfonate groups at the upper-rim of SCnA. Three main factors including the size of calixarene cavity, guest substituent, and pH collectively influence the binding affinity between SCnA and biguanidinium. From a view of thermodynamics, the complexation between SC4A and biguanidinium is dominantly driven by the enthalpy changes, while the complexation between SC5A and biguanidinium is driven almost equally by the enthalpy and entropy changes. Moreover, the complexation of SCnAs with guests is seriously influenced by surrounding ions which was confirmed by the ITC measurements in phosphate buffer.
|
Download:
|
| Fig. 2. The structures of sulfonatocalix[4, 5]arenes and biguanidiniums. | |
Another typical example was the potential application of p-sulfonatocalix[n]arene to treat viologen poisoning (Fig. 3) [23]. Viologen herbicides are used by millions of growers and more than 100 crops in over 120 countries and have high toxicity and longterm formidable risks to human health, society, and the environment [24, 25]. p-Sulfonatocalix[n]arenes were found to be capable of inhibiting the viologen toxicity. With the highly effective complexation of p-sulfonatocalix[n]arene, the reduction potential of viologen shifted to a more negative value which made the interactions of viologen with reducing agent in the cell and the generation of radical cation became more difficult. In the mice test, the mortality rate was found to be significantly decreased when the viologen-poisoned mice were dealed with p-sulfonatocalix[n] arene, and the paraquat-induced destruction of tissue structure was also prevented. These results pave a new therapeutic protocol based on host-guest chemistry for the effective treatment of viologen poisoning and made p-sulfonatocalix[n]arene a promising novel medicine.
|
Download:
|
| Fig. 3. Treatment of viologen poisoning by p-sulfonatocalixarenes. | |
The electron-rich anionic calixarenes were also good receptors for trimethylammonium-containing neurotransmitters [26-29]. The sensing of acetylcholine and its application to enzymatic reaction was realized according to the supramolecular tandem assay principle with the host-guest reporter pair of p-sulfonatocalix[n]arene (n = 4–5) and lucigenin (LCG), a cationic aromatic fluorescent dye (Fig. 4) [30]. The strong complexation between LCG and SC4A caused a strong static fluorescence quenching (factor 140) of LCG through an exergonic electron transfer process which made these host—guest pair a reporter pair for lable-free continuous real-time enzyme assays. These host-guest reporter pairs enable the tandem assay for direct monitoring acetylcholinesterase and cholineoxidase with low micro molar sensitivity. This method also enabled the acetylcholinesterase inhibitor and activator screening.
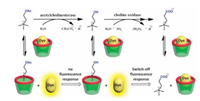
|
Download:
|
| Fig. 4. Tandem assays reactions of acetylcholinesterase and cholineoxidase. | |
Another example was a fluorescence-tunable hydrogel with white-light emitting property (Fig. 5) [31]. The hydrogel was constructed through free-redical copolymerization of acrylamide (AAm), 1-adamantyl acrylamide (AAmAd), 4-(allyloxy)sulfonatocalix[4]arene (SC4AA) and N, N-methylenebisacrylamide (bisAAm). The fragment of adamantyl (Ad) and sulfonatocalix[4]arene (SC4A) worked as binding sites to realize the orthogonal supramolecular recognition of β-cyclodextrin-modified tetraphenylethene (TPECD) and 4-[4-(dimethylamino)styryl]-1-methylpyridinium iodide (DASPI). Through the fluorescence enhancement and FRET effect induced by host–guest complexation, multi-color fluorescence emissions were achieved with the regulation from blue light to yellow light especially including white light. This post-modification method based on integrative self-sorting concept was a novel and convenient way to fabricate tunable fluorescent hydrogel with multi-color emissions and might have potential applications in organic luminescent displayers or optical devices.
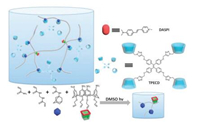
|
Download:
|
| Fig. 5. Orthogonal supramolecular recognition in the luminescent hydrogel. | |
Like calix[n]arene, pillar[n]arenes are another kind of macrocyclic host molecules with outstanding ability to selectively complex various guests. In particularly, water-soluble pillar[n] arenes have gained more and more attention for their applications in biomedical research such as cell imaging and drug delivery. A typical example was a turn-on fluorescence switch based on hostguest pair of cationic pillar[5]arene (AP5) and the pH-sensitive dye salicylaldehyde (Fig. 6) [32]. More interestingly, AP5 could decrease the pKa of salicylaldehyde in a complexation-induced dye deprotonation manner. The disaggregation of the assemblies resulted in a significant fluorescence enhancement of the dye. This turn-on fluorescence switch was further used for fluorescence indicator displacement (FID) detection of phenols and chlorophenols. The author believed that this FID system could be further used in the detection of biological and environmental related anionic compounds.
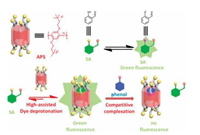
|
Download:
|
| Fig. 6. Fluorescence indicator displacement detection based on pillar[5]arene assisted dye deprotonation. | |
3. Multi-functional assembly
Compared with other water-soluble macrocycles, multicharged calixarenes and pillarenes have a unique property that could promote the aggregation of amphiphilic guest molecules. This unique property was early defined as "calixarene-induced aggregation (CIA)" with the following three aspects: 1) lower the critical aggregation concentration (CAC); 2) enhance the aggregate stability and compactness; 3) regulate the degree of order in the aggregates. Various guest molecules including fluorescent dyes, surfactants, drugs, and proteins were used to construct supramolecular assemblies with SCnA through the CIA principle to develop novel applications in drug-delivery, smart material and catalysis [33-43]. Hydrophobic/π-stacking interactions and electrostatic interactions are two major interactions serving for CIA. To the amphiphilic guest molecules themselves, hydrophobic/ π-stacking interactions between alkyl and aromatic moieties provide the initial power for self-organization in water. Meanwhile, electrostatic repulsion between cationic/anionic head groups prevented the formation of large aggregates. However, with the interactions with multi-charged calixarenes and pillarenes, the electrostatic repulsion between the head groups of guest molecules is replaced by the electrostatic attraction between the oppositely charged head groups of guests and host molecules. So, with the synergistic effects of hydrophobic/π-stacking interactions and electrostatic interactions, large multi-dimensional supramolecular assemblies by CIA were obtained. These assemblies formed through CIA were usually larger than the self-assemblies of guest molecules alone.
Vesicles are useful tools in chemistry, biology and material science. A supramolecular binary vesicle constructed from p-sulfonatocalix[5]arene (SC5A) and 1-pyrenemethylaminium (PMA) was reported (Fig. 7) [44]. With PMA alone, no organized assembly was observed. Interestingly, with the complexation towards p-sulfonatocalix[5]arene, vesicle-like nanoparticles were formed. Through the CIA principle, the CAC value of PMA lowered 3 times. These vesicles also possessed good thermal reversibility which made them a potential cargo delivery system. Furthermore, in another report, mono-p-sulfonatocalix[n]arene was replaced by bis-p-sulfonatocalix[n]arene and nanorods assemblies instead of vesicle was formed [45]. These two examples also proved the abilities of CIA assemblies in constructing nanomaterials with controllable morphologies.
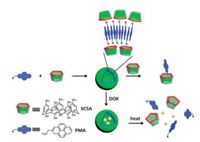
|
Download:
|
| Fig. 7. Supramolecular assembly constructed of SC5A and PMA. | |
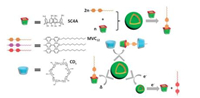
Besides the example of PMA, another amphiphilic guest molecule 1-methyl-10-dodecyl-4, 40-bipyridinium (MVC12) was also used to assemble with p-sulfonatocalix[4]arene (SC4A) to construct vesicle (Fig. 8) [46]. In this work, with the addition of SC4A, a significant decrease of CAC value of MVC12 as ~1000 times was observed. Meanwhile, these supramolecular binary vesicles can be tuned by multi stimulus such as temperature, reduction and another competition macrocycle, i.e., cyclodextrins (CDs). By the reduction of MVC12 to a radical cation state, smaller nanoparticles could be obtained. Moreover, the disassembly of vesicles could be realized through two-stepped reduction of MVC12 to a neutral form, rising the temperature, or the addition of CDs. These three stimuli-response disassembly could be used as effective switches to release the entrapped cargos. Doxorubicin (DOX) was chosen as a model molecule to test the drug delivery of this supramolecular vesicle. The corresponding cell experiment showed that this drug delivery system did not affect the therapeutic effect of DOX to cancer cells but reduced the damage to normal cells. This supramolecular binary vesicle formed by CIA could be a promising platform for controlled release and drug delivery.
|
Download:
|
| Fig. 8. Multi-stimuli response supramolecular binary vesicle composed of SC4A and asymmetric viologen. | |
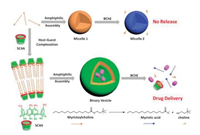
Since enzymes are great ingredients in living body and the aberrations in enzymes expression related to many diseases, the constructing of enzyme-responsive drug delivery nanomaterials gained a lot of interests. A typical example was an enzymeresponsive supramolecular vesicle constructed from SC4A and myristoylcholine through CIA principle (Fig. 9) [47]. Myristoylcholine was a substrate of butyrylcholinesterase (BChE) and also an amphiphilic molecule with the CAC values as 2.5 mol/L. Meanwhile, the CAC value of the hydrolysis product of myristoylcholine was 4.5 mol/L. However, SC4A can lower the CAC value of myristoylcholine by 100 times. More significantly, the overexpression of cholinesterase was an important symbol of Alzheimer's disease. For this reason, this supramolecular assembly had the possibility to be applied in targeted delivery system for anti-Alzheimer's disease drug.
|
Download:
|
| Fig. 9. Enzymatic responsiveness of amphiphilic assemblies of myristoylcholine with and without SC4A. | |
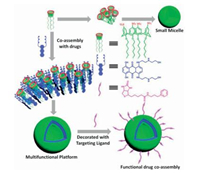
Another kind of water-soluble calixarene derivate was amphiphilic SCnA which has a similar hydrophilic macrocyclic skeleton and several hydrophobic alkyl chains at the lower rim. Different from CIA assemblies, amphiphilic SCnAs were employed to coassemble with amphiphilic drugs. Meanwhile, in these coassemblies, the cavities of macrocyclic hosts tended to locate on the surface of nanoparticle and remain affinity towards different kinds of guest molecules. The stability of this co-assembly could also be improved by the rigid cone-type skeleton of calixarene. Liu reported a supramolecular nanoparticle formed by the coassembly of chlorpromazine hydrochloride (CPZ) and amphiphilic SC4A4 (AMSC4A) (Fig. 10) [48]. Interestingly, through the recognition site of SC4A on the surface of AMSC4A/CPZ nanoparticle, trimethylated chitosan (TMC) could be modified to realize the targeting ability. Meanwhile this AMSC4A/CPZ nanoparticle could load irinotecan·HCl and mitoxantrone·HCl with high efficiency. This amphiphilic drug co-assembly strategy provides a novel platform for multi-functional drug delivery since various functional molecules can be easily modified on the nano-carrier through host-guest interactions.
|
Download:
|
| Fig. 10. Functional drug co-assembly by amphiphilic calixarene as "drug chaperone". | |
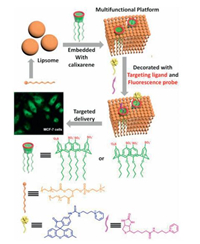
Liposome, a natural nano-vesicle formed by amphiphilic molecule, could load both hydrophilic and hydrophobic cargos in the internal aqueous environment and the membrane volume separately (Fig. 11) [49]. Several kind of functional molecules as imaging probes, targeting ligands, and treating modules, were modified on liposomes to achieve diagnostic and therapeutic purpose [50]. Among them, a typical example was a supramolecular drug delivery nano-vesicle formed by embedding AMSC4A on liposome (Fig. 11) [51]. Interestingly, AMSC4A could not only increase the stability of vesicles but also functionalize fluorescence image probes and targeting ligands by the cavities. Furthermore, the confocal laser scanning microscopic experiments proved that these supramolecular nano-vesicles could realize receptor-mediated internalization to cancer cells.
|
Download:
|
| Fig. 11. Multifunctional liposome and the noncovalent surface modification via amphiphilic calixarene. | |
Photolyzable assembly gained lots of attentions in the fields of degradable material, drug delivery vehicle, and tissue engineering. Anthracenes are typical photolyzable molecules with highly reactivity upon light irradiation (Fig. 12). Liu reported a photolyzable system constructed from an amphiphilic 9-alkoxy-substituted anthracene (AnPy) and SC4A [52]. Interestingly, SC4A could inhibit the fluorescence quenching of AnPy. This is the predominant condition for the photosensitization of Anpy since the fluorescence of photosensitizer was always self-quenched after aggregation and led to reduced singlet oxygen generation and photoreactivity. Due to the supramolecular assembly formed through CIA, the photodecomposition of AnPy could be greatly promoted. This photolyzable supramolecular amphiphilic assembly constructed from CIA was a meaningful enlightenment in the area of photodynamic therapy and photodegradation of pollutants.
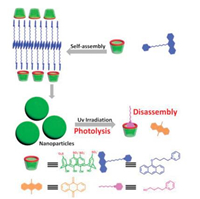
|
Download:
|
| Fig. 12. Photolyzable supramolecular amphiphilic assembly. | |
Another interesting example was a supramolecular selfassembly with near-infrared (NIR) fluorescence for cell imaging constructed from an anthracyl pyridinium guest molecule (ENDT) and two macrocycles as cucurbituril[8] (CB[8]) and amphiphilic SC4A (SC4AD) (Fig. 13) [53]. ENDT has very weak fluorescence emission at 625 nm due to the aggregation induced quenching (ACQ) effect. However, the fluorescence of ENDT greatly increased after assembled with CB[8] to form J-aggregation nanorods and the emission light red-shifted to 655 nm. More interestingly, the ENDT/CB[8] assemblies further assembled with SC4AD to form a spherical nanoparticle with further fluorescence enhancement. Such multi-stage supramolecular assembly undergoes two processes as host-guest complexation and calixarene induced aggregation. Furthermore, since the fluorescence emission of ENDT/CB[8]/SC4AD reached the NIR region, such nanoparticles were further used as targeted imaging agents for lysosome. This supramolecular multi-stage assembly brought a novel constructing strategy for NIR fluorescent cell imaging since it overcame the usual defect as aggregation induced quenching (ACQ) and nonnear infrared emission.
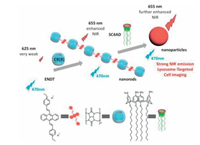
|
Download:
|
| Fig. 13. NIR fluorescent supramolecular assembly meditated by cucurbituril and amphiphilic calixarene. | |
Pillararenes were widely used to construct supramolecular assemblies [54-72]. Among the stimuli-responsive supramolecular nanomaterials, size switching was a valuable property since size is a basic and critical factor for materials. A typical example was a photo/thermal-stimuli-responsive supramolecular assembly based on an anionic pillar[5]arene (P5) and an azobenzene derivate guest (Gazo) (Fig. 14) [73]. In this work, both the trans- and cis-configuration of Gazo could fit in the cavity of P5 with similar affinity. This distinctive binding behavior of host-guest pair (P5/ Gazo) would be a useful property for constructing size swtichable nanomaterials. Since Gazo can respond to photo/thermal stimulation, the size of supramolecular assembly formed by Gazo with P5 can realized a reversible size change between 800 nm and 250 nm which also lead to a turbid-to-clear change of the solution. This size switchable supramolecular assembly based on P5 may have potent application in constructing control-release smart material.
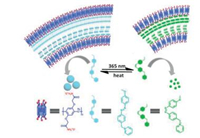
|
Download:
|
| Fig. 14. Photo/thermal-stimuli size switchable supramolecular assembly. | |
2D nanosheet is a desirable nanostructure with several irreplaceable properties as homogeneous features, large surface areas, and highly accessible active sites. The construction of 2D organicinorganic nanosheet based on supramolecular assembly was reported (Fig. 15) [74]. In this work, a phosphotungstic acid H3PW12O40 (POM) and a imidazolium functionalized pillar[6]arene (P6) was used to construct organic-inorganic nanosheet. Both transmission electron microscopy (TEM) and scanning electron microscopy (SEM) presented micrometer-scale 2D film morphology of P6/POM. Moreinterestingly, the obtained 2D sheets couldenhance the degradation efficiency of POM. The mechanism of accelerated degradation was explained as follow. In this P6/POM assembly, the POMs were separated by P6 through hydrogen-bonding and electrostatic interactions so that H2O2 could transit the cavities of P6 and arrive at the catalytic sites of POMs. These hybrid 2D films based on supramolecular assembly of pillarene may have potential enlightenment in dye degradations material.
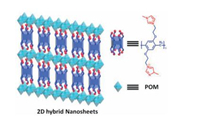
|
Download:
|
| Fig. 15. 2D organic–inorganic nanosheets constructed of pillar[6]arene and polyoxometalate. | |
4. Crosslinked muti-dimensional materials
Another interesting work was a tunable organic photoluminescent solid material. Sulfonatocalixarenes as macrocycles with specific pore structures and selective binding abilities have been used for fabricating tunable solid state photo-luminescent materials (Fig. 16) [75]. In this work, a mesoporous polymer based on SC4A and TPE have been prepared with certain absorption capacity for 4-[4-(dimethylamino)styryl]-1-methylpyridinium iodide (DASPI) through host–guest complexation. As DASPI was captured by SC4A cavity, which spatially separated with the TPE, a strong FRET effect could happen between TPE and DASPI. Due to the good overlap between the emission of TPE and the excitation of DASPI, the efficiency of energy transfer was 87%. This strategy through FRET between crosslinked macrocyclic host polymers and fluorescent guests to realize tunable photo-luminescence may develop into a platform for constructing solid photo-luminescence-tunable materials.
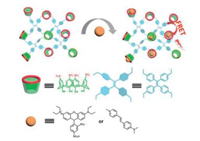
|
Download:
|
| Fig. 16. Fluorescent porous materials based on sulfonatocalixarenes. | |
Since the complexation between sulfonatocalixarenes and dyes has been systematically studied, sulfonatocalixarene became an effective candidate material for the removal of organic pollutants from waste water. Mesoporous polymer films based on crosslinked SC4A and P5A were prepared through interfacial polymerizations (Fig. 17) [76]. By incorporating new binding sites to mesoporous materials, the adsorptive polymer material has two types of pores: one type belong to the polymer network which allows a fast flow rate of solvents, the other belong to the cavity of macrocycle which traps small organic dyes. Owing to these two kinds of pores, the polymers can effectively remove anionic and cationic dyes but remain high permeability. Furthermore, cationic and anionic dyes can be separated by the corresponding films due to the selective host-guest molecular recognition. This work set up a new fabricating strategy for fast and efficient separations.
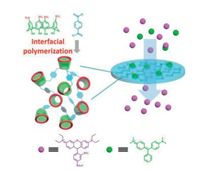
|
Download:
|
| Fig. 17. Crosslinked sulfonatocalixarene polymers for ultrafast separation of organic dyes. | |
5. Summary and outlook
Since the synthetic technique of water-soluble calixarene/ pillararene gained great developments, abundant applications based on these water-soluble macrocycles were explored in the area of recognition and sensing, stimuli-responsive assembly, drug delivery, cell-imaging, light degradation and so on. Base on the current research, more efforts should be contributed to the following directions: 1) finding more biologically related molecules with affinities toward water-soluble calixarene/pillararene; 2) realizing the precise control of assembly in size, morphology and stability; 3) finding other stimuli-response measures; 4) exploring the application of calixarene/pillararene-based functional materials in other areas.
AcknowledgmentWe thank the National Natural Science Foundation of China (Nos. 21672113, 21432004, 21772099, 21861132001) for financial support.
| [1] |
Y. Wang, H. Xu, X. Zhang, Adv. Mater. 21 (2009) 2849-2864. DOI:10.1002/adma.v21:28 |
| [2] |
W. Pisula, M. Kastler, D. Wasserfallen, et al., Angew. Chem. Int. Ed. 45 (2006) 819-823. DOI:10.1002/(ISSN)1521-3773 |
| [3] |
C. Wang, S. Yin, S. Chen, et al., Angew. Chem. Int. Ed. 47 (2008) 9049-9052. DOI:10.1002/anie.v47:47 |
| [4] |
X. Zhang, S. Rehm, Safont-Sempere M.M., F. Würthner, Nat. Chem. 1 (2009) 623. DOI:10.1038/nchem.368 |
| [5] |
Y. Wang, N. Ma, Z. Wang, X. Zhang, Angew. Chem. Int. Ed. 46 (2007) 2823-2826. DOI:10.1002/(ISSN)1521-3773 |
| [6] |
K. Kim, W.S. Jeon, J.K. Kang, et al., Angew. Chem. Int. Ed. 42 (2003) 2293-2296. DOI:10.1002/anie.200250692 |
| [7] |
N. Kimizuka, T. Kawasaki, T. Kunitake, J. Am. Chem. Soc. 115 (1993) 4387-4388. DOI:10.1021/ja00063a077 |
| [8] |
N. Kimizuka, T. Kawasaki, K. Hirata, T. Kunitake, J. Am. Chem. Soc. 120 (1998) 4094-4104. DOI:10.1021/ja974379+ |
| [9] |
X. Zhang, Z. Chen, F. Würthner, J. Am. Chem. Soc. 129 (2007) 4886-4887. DOI:10.1021/ja070994u |
| [10] |
V. Balzani, M. Gómez-López, J.F. Stoddart, Acc. Chem. Res. 31 (1998) 405-414. DOI:10.1021/ar970340y |
| [11] |
M.J. Blanco, M. Consuelo Jiménez, J.C. Chambron, et al., Chem. Soc. Rev. 28 (1999) 293-305. DOI:10.1039/a901205b |
| [12] |
J.P. Sauvage, Acc. Chem. Res. 31 (1998) 611-619. DOI:10.1021/ar960263r |
| [13] |
Y. Liu, M. Han, H.Y. Zhang, L.X. Yang, W. Jiang, Org. Lett. 10 (2008) 2873-2876. DOI:10.1021/ol801048t |
| [14] |
M. Suresh, A.K. Mandal, E. Suresh, A. Das, Chem. Sci. 4 (2013) 2380-2386. DOI:10.1039/c3sc50282a |
| [15] |
H. Xu, D.M. Rudkevich, J. Org. Che. 69 (2004) 8609-8617. DOI:10.1021/jo0488210 |
| [16] |
S. Shinkai, S. Mori, T. Tsubaki, T. Sone, O. Manabe, Tetrahedron Lett. 25 (1984) 5315-5318. DOI:10.1016/S0040-4039(01)81592-6 |
| [17] |
S. Shinkai, K. Araki, T. Matsuda, et al., J. Am. Chem. Soc. 112 (1990) 9053-9058. DOI:10.1021/ja00181a004 |
| [18] |
Y. Liu, D.S. Guo, H.Y. Zhang, Y.H. Ma, E.C. Yang, J. Phy. Chem. B 110 (2006) 3428-3434. DOI:10.1021/jp0545703 |
| [19] |
H.X. Zhao, D.S. Guo, Y. Liu, J. Phy. Chem. B 117 (2013) 1978-1987. DOI:10.1021/jp312744d |
| [20] |
G.S. Wang, H.Y. Zhang, D. Li, P.Y. Wang, Y. Liu, Supramol.Chem. 23 (2011) 441-446. DOI:10.1080/10610278.2010.544736 |
| [21] |
G.S. Wang, H.Y. Zhang, F. Ding, Y. Liu, J. Inclusion Phenom. Macrocyclic Chem. 69 (2011) 85-89. DOI:10.1007/s10847-010-9817-1 |
| [22] |
D.S. Guo, H.Q. Zhang, F. Ding, Y. Liu, Org. Biomol. Chem. 10 (2012) 1527-1536. DOI:10.1039/c2ob06313a |
| [23] |
K. Wang, D.S. Guo, H.Q. Zhang, et al., J. Med. Chem. 52 (2009) 6402-6412. DOI:10.1021/jm900811z |
| [24] |
J.M. Hatcher, K.D. Pennell, G.W. Miller, Trends Pharmacol. Sci. 29 (2008) 322-329. DOI:10.1016/j.tips.2008.03.007 |
| [25] |
R.J. Dinis-Oliveira, F. Remião, H. Carmo, et al., Neurotoxicology 27 (2006) 1110-1122. DOI:10.1016/j.neuro.2006.05.012 |
| [26] |
H. Bakirci, W.M. Nau, Adv. Funct. Mater. 16 (2006) 237-242. DOI:10.1002/(ISSN)1616-3028 |
| [27] |
T. Jin, Sensors 10 (2010) 2438-2449. DOI:10.3390/s100302438 |
| [28] |
T. Jin, F. Fujii, Y. Ooi, Sensors 8 (2008) 6777-6790. DOI:10.3390/s8106777 |
| [29] |
K.N. Koh, K. Araki, A. Ikeda, H. Otsuka, S. Shinkai, J. Am. Chem. Soc. 118 (1996) 755-758. DOI:10.1021/ja951488k |
| [30] |
D.S. Guo, V.D. Uzunova, X. Su, Y. Liu, W.M. Nau, Chem. Sci. 2 (2011) 1722-1734. DOI:10.1039/c1sc00231g |
| [31] |
Q. Zhao, Y. Chen, S.H. Li, Y. Liu, Chem. Commun. 54 (2018) 200-203. DOI:10.1039/C7CC08822A |
| [32] |
B. Hua, L. Shao, G. Yu, F. Huang, Chem. Commun. 52 (2016) 10016-10019. DOI:10.1039/C6CC04919B |
| [33] |
D.S. Guo, K. Chen, H.Q. Zhang, Y. Liu, Chem. -Asian J. 4 (2009) 436-445. DOI:10.1002/asia.v4:3 |
| [34] |
D.S. Guo, B.P. Jiang, X. Wang, Y. Liu, Org. Biomol. Chem. 10 (2012) 720-723. DOI:10.1039/C2OB06973C |
| [35] |
O. Varga, M. Kubinyi, T. Vidóczy, et al., J. Photochem. Photobiol. A:Chem. 207 (2009) 167-172. DOI:10.1016/j.jphotochem.2009.07.001 |
| [36] |
V. Lau, B. Heyne, Chem. Commun. 46 (2010) 3595-3597. DOI:10.1039/c002128h |
| [37] |
M. Megyesi, L. Biczók, J. Phy. Chem. B 114 (2010) 2814-2819. DOI:10.1021/jp910418k |
| [38] |
N. Basílio, Á. Piñeiro, J.P. Da Silva, L. García-Río, J. Org. Chem. 78 (2013) 9113-9119. DOI:10.1021/jo401307c |
| [39] |
N. Basilio, L. García-Río, Chem.-Eur. J. 15 (2009) 9315-9319. DOI:10.1002/chem.v15:37 |
| [40] |
V. Francisco, N. Basilio, L. Garcia-Rio, et al., Chem. Commun. 46 (2010) 6551-6553. DOI:10.1039/c0cc01806f |
| [41] |
N. Basilio, B. Gómez, L. Garcia-Rio, V. Francisco, Chem.-Eur. J. 19 (2013) 4570-4576. DOI:10.1002/chem.201203377 |
| [42] |
N. Basilio, M. Martín-Pastor, L. García-Río, Langmuir 28 (2012) 6561-6568. DOI:10.1021/la3006794 |
| [43] |
V. Wintgens, C. Le Coeur, C. Amiel, et al., Langmuir 29 (2013) 7682-7688. DOI:10.1021/la401185p |
| [44] |
K. Wang, D.S. Guo, Y. Liu, Chem.-Eur. J. 16 (2010) 8006-8011. DOI:10.1002/chem.201000991 |
| [45] |
K. Wang, D.S. Guo, Y. Liu, Chem.-Eur. J. 18 (2012) 8758-8764. DOI:10.1002/chem.v18.28 |
| [46] |
K. Wang, D.S. Guo, X. Wang, Y. Liu, ACS Nano 5 (2011) 2880-2894. DOI:10.1021/nn1034873 |
| [47] |
D.S. Guo, K. Wang, Y.X. Wang, Y. Liu, J. Am. Chem. Soc. 134 (2012) 10244-10250. DOI:10.1021/ja303280r |
| [48] |
Y.X. Wang, D.S. Guo, Y.C. Duan, Y.J. Wang, Y. Liu, Sci. Rep. 5 (2015) 9019. DOI:10.1038/srep09019 |
| [49] |
A. Rösler, G.W.M. Vandermeulen, H.A. Klok, Adv. Drug Deliv. Rev. 53 (2001) 95-108. DOI:10.1016/S0169-409X(01)00222-8 |
| [50] |
F. Gu, L. Zhang, B.A. Teply, et al., Proc. Nat. Acad. Sci. 105 (2008) 2586. DOI:10.1073/pnas.0711714105 |
| [51] |
Y.X. Wang, Y.M. Zhang, Y.L. Wang, Y. Liu, Chem. Mater. 27 (2015) 2848-2854. DOI:10.1021/cm504653k |
| [52] |
Y.X. Wang, Y.M. Zhang, Y. Liu, J. Am. Chem. Soc. 137 (2015) 4543-4549. DOI:10.1021/jacs.5b01566 |
| [53] |
X.M. Chen, Y. Chen, Q. Yu, B.H. Gu, Y. Liu, Angew. Chem. Inter. Ed. 57 (2018) 12519-12523. DOI:10.1002/anie.201807373 |
| [54] |
G. Yu, X. Zhou, Z. Zhang, et al., J. Am. Chem. Soc. 134 (2012) 19489-19497. DOI:10.1021/ja3099905 |
| [55] |
X. Chi, G. Yu, L. Shao, J. Chen, F. Huang, J. Am. Chem. Soc. 138 (2016) 3168-3174. DOI:10.1021/jacs.5b13173 |
| [56] |
G. Yu, W. Yu, L. Shao, et al., Adv. Funct. Mater. 26 (2016) 8999-9008. DOI:10.1002/adfm.v26.48 |
| [57] |
K. Jie, Y. Zhou, Y. Yao, B. Shi, F. Huang, J. Am. Chem. Soc. 137 (2015) 10472-10475. DOI:10.1021/jacs.5b05960 |
| [58] |
G. Yu, J. Zhou, J. Shen, G. Tang, F. Huang, Chem. Sci. 7 (2016) 4073-4078. DOI:10.1039/C6SC00531D |
| [59] |
G. Yu, W. Yu, Z. Mao, C. Gao, F. Huang, Small 11 (2015) 919-925. DOI:10.1002/smll.v11.8 |
| [60] |
X. Chi, X. Ji, D. Xia, F. Huang, J. Am. Chem. Soc. 137 (2015) 1440-1443. DOI:10.1021/ja512978n |
| [61] |
B. Shi, K. Jie, Y. Zhou, et al., J. Am. Chem. Soc. 138 (2016) 80-83. DOI:10.1021/jacs.5b11676 |
| [62] |
X.Y. Hu, L. Gao, S. Mosel, et al., Small 14 (2018) 1803952. DOI:10.1002/smll.v14.52 |
| [63] |
M. Zuo, W. Qian, T. Li, et al., ACS Appl. Mater. Interfaces 10 (2018) 39214-39221. DOI:10.1021/acsami.8b14110 |
| [64] |
M. Zuo, W. Qian, Z. Xu, et al., Small 14 (2018) 1801942. DOI:10.1002/smll.v14.38 |
| [65] |
S. Guo, Y. Song, Y. He, X.Y. Hu, L. Wang, Angew.Chem.Inter.Ed. 57 (2018) 3163-3167. DOI:10.1002/anie.201800175 |
| [66] |
X. Liu, W. Shao, Y. Zheng, et al., Chem. Commun. 53 (2017) 8596-8599. DOI:10.1039/C7CC04932C |
| [67] |
X. Liu, K. Jia, Y. Wang, et al., ACS Appl. Mater. Interfaces 9 (2017) 4843-4850. DOI:10.1021/acsami.7b00643 |
| [68] |
J.R. Wu, Y.W. Yang, Chem. Commun. 55 (2019) 1533-1543. DOI:10.1039/C8CC09374A |
| [69] |
T. Xiao, W. Zhong, L. Zhou, et al., Chin. Chem. Lett. 30 (2019) 31-36. DOI:10.1016/j.cclet.2018.05.034 |
| [70] |
T. Xiao, L. Zhou, L. Xu, et al., Chin. Chem. Lett. 30 (2019) 271-276. DOI:10.1016/j.cclet.2018.05.039 |
| [71] |
Y. Zhou, H. Li, Y.W. Yang, Chin. Chem. Lett. 26 (2015) 825-828. DOI:10.1016/j.cclet.2015.01.038 |
| [72] |
N. Song, T. Kakuta, T.a. Yamagishi, Y.W. Yang, T. Ogoshi, Chemistry 4 (2018) 2029-2053. DOI:10.1016/j.chempr.2018.05.015 |
| [73] |
C.C. Zhang, S.H. Li, C.F. Zhang, Y. Liu, Sci. Rep. 6 (2016) 37014. DOI:10.1038/srep37014 |
| [74] |
N. Cheng, Y. Chen, X. Wu, Y. Liu, Chem. Commun. 54 (2018) 6284-6287. DOI:10.1039/C8CC03306D |
| [75] |
Q. Zhao, Y. Liu, Chem. Commun. 54 (2018) 6068-6071. DOI:10.1039/C8CC03461C |
| [76] |
Q. Zhao, Y. Liu, Chem. Commun. 54 (2018) 7362-7365. DOI:10.1039/C8CC04080J |
 2019, Vol. 30
2019, Vol. 30 

