b Center of Analysis and Testing, Nanchang University, Nanchang 330031, China;
c School of Chemistry, Nanchang University, Nanchang 330031, China
Plant growth regulators (PGRs) are small molecule compounds that affect growth and development of plants [1, 2]. PGRs are commonly divided into three categories including growth promoters, growth retardants and growth inhibitors. These compounds play an important role in enhancing plant resistance, promoting the division and growth of plant cells, increasing yield, improving quality, and making great contributions to agricultural production and development [3, 4]. However, overuse of PGRs in agriculture has resulted in their remaining in crops and great potential hazards to humans. Therefore, the determination of PGRs in edible oils is very important.
Until now, many PGRs detection methods of various food samples have been reported, such as liquid chromatography-mass spectrometry [5-7] and gas chromatography-mass spectrometry [8]. However, high requirements on sensitivity of instruments and experimental cost obviously increased. As majority of PGRs were trace amount level in complicated food matrix, some methods based on modified QuEChERS methods [9], new nanoparticles and materials [10-12], extracting and enrichment techniques [13, 14] were sprout out continuously. Those promising techniques were not only focused on improving precision, sensitivity, selectivity and accuracy of method, but also declared time-saving and convenience to carry out. Thus, with great advantage of rapid separation, controllable shape, size and structure, modified magnetic separation has outperformed in the sample pretreatment of food, environment, and so on [15-23]. Xu et al. [24] reported a magnetic solid-phase extraction based on Fe3O4-NH2@GO@DES material for the extraction of proteins. The analytical results demonstrated that the prepared magnetic nanoparticles did have extraction ability on proteins in bovine whole blood.
Deep eutectic solvent (DES), as we all know, was first introduced by Abbot et al. in 2003 and has gradually been considered as a new type of green ionic liquid [25, 26]. Compared with traditional organic solvents and ionic liquids, DES was used in advanced research [24, 27-29] as well as in industry [30, 31] due to their remarkable physicochemical properties [25, 32-34]. A DESbased liquid-phase microextraction method was established to detect several acidulous plant growth regulators in edible vegetable oils, including indole-3-acetic acid, indole-3-butyric acid and 4-iodophenoxyacetic acid [35]. However, we found it is difficult to separate DES reagent and oil matrix through the bald way of withdrawing with microliter syringe.
Herein, in this work, we established a new facile and effective method for further investigation of trace amount PGRs by developing novel DES-Fe3O4 composites. Five typical PGRs in a cash crop growth cycle, including indole-3-acetic acid, abscisic acid, thidiazuron, 1- naphthylacetic acid and forchlorfenuron were adopted.
Structures of five plant growth regulators (Fig. S1 in Supporting information), solutions and material preparation methods, movement of DES-Fe3O4 composite (Fig. S2 in Supporting information) and extraction efficiency calculated equations can be found in Supporting information.
Extraction procedure: Take 10 mL working solution in a centrifuge tube, and 40 μL DES-Fe3O4 was transferred into the same centrifuge tube, then ultrasound in a water bath at 50 ℃ for 30 min. After ultrasound, the composite was gathered together by an external magnet. Then, the composite can be held firmly along with the magnet. Next, we took the centrifuge tube upright, and moved the magnet very fast from the bottom of the tube to the top. Interestingly, Fe3O4 can move along with the magnet, but DES was dropped to the bottom of tube due to its own gravity. After removed from Fe3O4, 3 μL DES was drawn by syringe and injected into liquid chromatography for analysis.
Chromatographic performance: Liquid chromatography was carried out in an Agilent 1260 system equipped with quaternary pump, column oven, sample injector and ultra violet detector. The whole chromatographic separation was performed on a C18 silica gel column (250 mm × 4.6 mm i.d.) set at 30 ℃. Mobile phase were mixtures of 60% methanol and 40% water acidified with 0.2% formic acid (v/v) at a flow rate of 0.6 mL/min. In addition, UV detection was performed at a wavelength of 255 nm and injected volume was 3 μL.
Studied materials were prepared and characterized. Scan election microscopy (SEM) and transmission election microscopy (TEM) images showed that synthesized Fe3O4 had spherical morphology (Figs. 1A and B). The particle size was less than 500 nm and had porous surface properties. X-ray diffraction pattern was shown in Fig. 1C. Obvious diffraction peaks (2θ) of Fe3O4 in this work at 30.18, 35.58, 43.098, 53.48, 56.958 and 62.838 were in accordance with those peaks in JCPDS card 72–23034 (220), (311), (400), (422), (440) and (511). It realized the structure of Fe3O4 product was cubic spinel like. Fig. 1D illustrated infrared characteristics of Fe3O4, DES and DES-Fe3O4. 582 cm-1 was characteristics of Fe-O vibration peak, 2939 cm-1 and 2883 cm-1 were C–H stretching region of - CH3 and - CH2, 3000 - 3600 cm-1 was a wide peak including stretching region of O- -H and N- -H. It demonstrated stretching region of C=O (1700 cm-1), C–H (1400 cm-1), C–O (1200 cm-1) and C–N (1041 cm-1), respectively. Furthermore, infrared characteristics of 1560 and 3445 cm-1 demonstrated a strong hydrogen bond in DES which was consisted of choline chloride and acetic acid.

|
Download:
|
| Fig. 1. Characterization of materials. (A) SEM image of Fe3O4, scale bar is 1 μm; (B) TEM image of Fe3O4, scale bar is 200 nm; (C) XRD pattern of Fe3O4; (D) The infrared characteristics of Fe3O4 (a), DES (b) and DES-Fe3O4 composite (c). | |
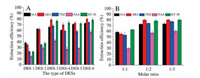
The extraction effects of five plant growth regulators by different DESs were studied as shown in Fig. 2. In Fig. 2A, it was shown that DES-1 and DES-2 offered lower extraction efficiency of all five plant growth regulators, comparing with other four types of DESs. Among DES-3, DES-4, DES-5 and DES-6, IAA, ABA, TDZ and KT-30 were well extracted, most of which achieved extraction efficiency between 60% and 80%. For NAA, DES-6 formed by ChCl and acetic acid can give a relatively higher extraction efficiency than other compositions. Finally, DES-6 forming by ChCl and acetic acid compounds was chosen as proper extractant for its better extraction efficiencies of five plant growth regulators.
|
Download:
|
| Fig. 2. Optimization of (A) DES type and (B) molar ratio. | |

|
Download:
|
| Fig. 3. Optimization of (A) extraction temperature, (B) extraction time and (C) the volume of DES. | |
Then proper molar ratio of DES-6 was further investigated as shown in Fig. 2B. Considering stability, viscosity and feasibility of DES, the molar ratio of ChCl and acetic acid changed from 1:1 to 1:3. As the amount of acetic acid was gradually increased, the viscosity of DES decreased. It is most likely that viscosity of DES significantly influences the effect of mass transfer and further influence the extraction effect. So, from experimental results, it was obviously seen that similar higher efficiencies of five analytes were obtained between 1:2 and 1:3. Molar ratio of polyols and carboxylic acid was finally fixed at 1:3. Optimization experiments of DES type and molar ratio illustrated that the purpose of simultaneous separation and enrichment can be successfully fulfilled through forming a two-phase system between DES and sample matrix.
Temperature affects the availability of mass transfer between DES and sample phase. It can directly affect DES extraction efficiency. In this study, considering the volatility and boiling point of n-hexane, the temperature range of ultrasonic extraction process was investigated from 20 ℃ to 55 ℃. As shown in Fig. 3A, extraction efficiencies of five plant growth regulators raised obviously from the lowest (25% at 20 ℃) to highest (85% at 50 ℃), and DES-6 extraction efficiency of IAA, ABA, TDZ and KT-30 were much higher than that of NAA from start to finish. During 50 - 55 ℃, the efficiency kept stable for most of five analytes. Thus, 50 ℃ was considered to be the optimal temperature of DES-6 in liquid phase microextraction for IAA, ABA, TDZ, NAA and KT-30.
In general, each extraction process is dynamic, and it is quite important to provide enough time for full extraction process. In this study, extraction time from 20 min to 55 min was investigated, and the result was shown in Fig. 3B. From the very beginning at 20 min, IAA, ABA, TDZ and KT-30 performed relatively high extraction efficiency, whereas the efficiency of NAA was only 50%. However, extraction efficiency of NAA rose up to nearly 80% and that of the other four plant growth regulators were all about 90% at 30 min. The results were kept even when extraction process lasted for 55 min. So, the optimal extraction time was fixed at 30 min.
As shown in Fig. 3C, DES-6 (20, 30, 40, 50 and 60 mL) was exposed to a fixed 5 mL of n-hexane working solution. The extraction efficiencies of IAA, ABA, TDZ, NAA and KT-30 increased with increasing amount of DES-6 between 20 μL and 40 μL, but it slightly decreased between 40 μL and 60 μL. It confirmed that extraction efficiency of unsaturated state rose up with increasing of extractant volume, and efficiency reduced after reaching saturation. Therefore, 40 μL of DES-6 achieved best extraction efficiency than other tested volumes.
In the experiment, effects of different compositions of mobile phase, such as methanol-acetonitrile, methanol-water, and methanol-water (0.2% acetic acid solution) on five plant growth regulators were investigated separately. The results showed that a better performance was obtained when methanol-water (0.2% acetic acid solution) (60/40, v/v) was applied as mobile phase and the whole time for one-round analysis was in 15 min. UV detector was used to determine standard solution of IAA, ABA, TDZ, NAA and KT-30, each maximum absorption wavelength of which were 220 nm, 265 nm, 290 nm, 220 nm and 265 nm, respectively. Considering both of sensitivity and accuracy, simultaneous detection wavelength of five plant growth regulators was chosen at 255 nm.
Without any extraction and enrichment steps, it was quite difficult to obtain the accurate and reliable results for the trace amount PGRs in the complicated oil matrix. However, the situation changed for the better. Based on the standard retention time, qualitative analysis of five PGRs in real oil samples was feasible, and the response intensities increased dramatically after efficient extraction and enrichment by DES. On the basis of optimized conditions, the validity of proposed method was examined by conducting a set of similar experiments at concentration levels in the range of 0.10–50 mg/mL for IAA, 0.06–50 mg/mL for ABA, 0.20– 50 mg/mL for TDZ, 0.50–50 mg/mL for NAA and 0.06–50 mg/mL for KT-30, respectively. And linear correlation factors (r) were 0.9997, 0.9998, 0.9997, 0.9995 and 0.9995. The results were listed in Table S2 (Supporting information). A repeatability study involved with three parallel replicated extractions was done, and relative standard deviations (RSDs) were 2.12%, 2.24%, 1.97%, 2.74%, and 1.75%, separately. Under the optimal extraction conditions, the limits of detection (LODs) were 0.10–2.00 ng/L, which the LODs were defined as amount of tested compound that generated a signal-to-noise (S/N) ratio of 3. As it can be seen that LOD values were much higher than that obtained without extraction. The proposed method was applied to determine the contents of IAA, ABA, TDZ, NAA and KT-30 in edible vegetable oils with RSDs (n = 3) less than 3.0%.
Different concentrations of mixed standard solution were added to five oil samples, respectively. The results were shown in Table S3 (Supporting information), recoveries of five plant growth regulators were ranged from 70.4% to 102.2%, indicating that DES-Fe3O4 based liquid phase microextraction method was effective.
In summary, the utilization of DES-Fe3O4 composite for the extraction of plant growth regulators from edible oil samples were described in this work. The incorporation of Fe3O4 microspheres facilitated the separation of the deep eutectic solvent from the sample matrix after extraction. Thus, the efficiency of sample pretreatment was improved. And the factors determining the extraction efficiencies, including the composition of the deep eutectic solvent, the temperature, the extraction time, and the volume of the deep eutectic solvent, were thoroughly investigated. This work explores the potential of DES-Fe3O4 composite for rapid extraction in food analysis.
AcknowledgmentsWe are grateful to the Research Project of Technology Program of Jiangxi Province (No. 20141BBG70093), State Key Laboratory of Food Science and Technology, Nanchang University (Nos. SKLFZZB-201718, SKLF-ZZA-201612), the National Natural Science Foundation of China (No. 21563020) and China Scholarship Council for financial support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.03.057.
| [1] |
A.E. Giannakoula, I.F. Ilias, J.J. Dragišić Maksimović, V.M. Maksimović, B.D. Živanović, J. Food Anal. 28 (2012) 46-53. DOI:10.1016/j.jfca.2012.06.005 |
| [2] |
L. Satish, A.S. Rency, P. Rathinapriya, et al., Plant Cell Tissue Org. 124 (2015) 15-31. |
| [3] |
S. Fahad, S. Hussain, S. Saud, et al., J. Agron. Crop Sci. 202 (2016) 139-150. DOI:10.1111/jac.2016.202.issue-2 |
| [4] |
S. Khatun, T. Roy, M. Haque, B. Alamgir, Int. J. Plant Soil Sci. 11 (2016) 1-9. |
| [5] |
N. Chamkasem, J. Agric. Food Chem. 65 (2017) 7535-7541. DOI:10.1021/acs.jafc.7b02419 |
| [6] |
K.G. Kim, D.W. Park, G.R. Kang, Food Chem. 208 (2016) 239-244. DOI:10.1016/j.foodchem.2016.04.002 |
| [7] |
X. Wang, X. Mao, A. Yan, et al., Food Anal. Method 9 (2016) 3268-3277. DOI:10.1007/s12161-016-0512-8 |
| [8] |
H. Chen, G. Gao, P. Liu, et al., Food Anal. Method 9 (2016) 2374-2384. DOI:10.1007/s12161-016-0427-4 |
| [9] |
N.C. Muñoz, L. Floriano, M.P. de Souza, et al., Food Anal. Method 10 (2016) 320-329. |
| [10] |
L. Hao, X.L. Liu, J.T. Wang, et al., Chin. Chem. Lett. 27 (2016) 783-788. DOI:10.1016/j.cclet.2016.01.021 |
| [11] |
Y. Su, H. Jiang, Y. Zhu, et al., J. Mater. Chem. A 2 (2014) 7281-7287. DOI:10.1039/C4TA00029C |
| [12] |
Z.S. Wu, S. Yang, Y. Sun, et al., J. Am. Chem. Soc. 134 (2012) 9082-9085. DOI:10.1021/ja3030565 |
| [13] |
A. Mirabi, Z. Dalirandeh, A.S. Rad, J. Magn. Magn. Mater. 381 (2015) 138-144. DOI:10.1016/j.jmmm.2014.12.071 |
| [14] |
J.L. Tadeo, C. Sánchez-Brunete, B. Albero, A.I. García-Valcárcel, J. Chromatogr. A 1217 (2010) 2415-2440. DOI:10.1016/j.chroma.2009.11.066 |
| [15] |
X. Cao, J. Chen, X. Ye, et al., J. Sep. Sci. 36 (2013) 3579-3585. DOI:10.1002/jssc.v36.21-22 |
| [16] |
H.L. Ding, Y.X. Zhang, S. Wang, et al., Chem. Mater. 24 (2012) 4572-4580. DOI:10.1021/cm302828d |
| [17] |
A.Q. Gao, H. Liu, L. Hu, et al., Chin. Chem. Lett. 29 (2018) 1301-1304. DOI:10.1016/j.cclet.2017.11.040 |
| [18] |
R. Ge, X. Li, M. Lin, et al., ACS Appl. Mater. Interfaces 8 (2016) 22942-22952. DOI:10.1021/acsami.6b07997 |
| [19] |
M. Iranmanesh, J. Hulliger, Chem. Soc. Rev. 46 (2017) 5925-5934. DOI:10.1039/C7CS00230K |
| [20] |
M. Jabbari, H. Razmi, S. Farrokhzadeh, Chromatographia 79 (2016) 985-993. DOI:10.1007/s10337-016-3117-x |
| [21] |
F. Liu, F. Niu, N. Peng, Y. Su, Y. Yang, RSC Adv. 5 (2015) 18128-18136. DOI:10.1039/C4RA15968C |
| [22] |
J. Shan, L. Wang, H. Yu, et al., Mater. Sci. Technol. 32 (2016) 602-614. |
| [23] |
M. Wierucka, M. Biziuk, TrAC-Trends Anal. Chem. 59 (2014) 50-58. DOI:10.1016/j.trac.2014.04.007 |
| [24] |
K. Xu, Y. Wang, X. Ding, et al., Talanta 148 (2016) 153-162. DOI:10.1016/j.talanta.2015.10.079 |
| [25] |
Y.T. Liu, Y.A. Chen, Y.J. Xing, Chin. Chem. Lett. 25 (2014) 104-106. DOI:10.1016/j.cclet.2013.09.004 |
| [26] |
L.X. Zhang, H. Yu, H.B. Yu, Z. Chen, L. Yang, Chin. Chem. Lett. 25 (2014) 1132-1136. DOI:10.1016/j.cclet.2014.03.029 |
| [27] |
M.R. Bhosle, L.D. Khillare, S.T. Dhumal, R.A. Mane, Chin. Chem. Lett. 27 (2016) 370-374. DOI:10.1016/j.cclet.2015.12.005 |
| [28] |
X. Li, K.H. Row, J. Sep. Sci. 39 (2016) 3505-3520. DOI:10.1002/jssc.201600633 |
| [29] |
S. Liang, H. Yan, J. Cao, et al., Anal. Chim. Acta 951 (2017) 68-77. DOI:10.1016/j.aca.2016.11.009 |
| [30] |
A. Shishov, A. Bulatov, M. Locatelli, S. Carradori, V. Andruch, Microchem. J. 135 (2017) 33-38. DOI:10.1016/j.microc.2017.07.015 |
| [31] |
E.L. Smith, A.P. Abbott, K.S. Ryder, Chem. Rev. 114 (2014) 11060-11082. DOI:10.1021/cr300162p |
| [32] |
C. Bakirtzi, K. Triantafyllidou, D.P. Makris, J. Appl. Res. Med. Arom. Plants 3 (2016) 120-127. |
| [33] |
A. García, E. Rodríguez-Juan, G. Rodríguez-Gutiérrez, J.J. Rios, J. FernándezBolaños, Food Chem. 197 (2016) 554-561. DOI:10.1016/j.foodchem.2015.10.131 |
| [34] |
W. Liu, K. Zhang, Y. Qin, J. Yu, Anal. Methods 9 (2017) 4184-4189. DOI:10.1039/C7AY01033H |
| [35] |
T. Tan, Z. Li, X. Mao, Y. Wan, H. Qiu, Anal. Methods 8 (2016) 3511-3516. DOI:10.1039/C6AY00053C |
 2019, Vol. 30
2019, Vol. 30 

