b College of Chemistry, Nanchang University, Nanchang 330088, China;
c The National Engineering Research Center for Bioengineering Drugs and Technologies, Institute of Translational Medicine, Nanchang University, Nanchang 330088, China;
d Department of Orthopedic Surgery, The Affiliated Stomatological Hospital of Nanchang University, The Key Laboratory of Oral Biomedicine, Nanchang 330006, China;
e Department of Orthopedic Surgery, The Second Affiliated Hospital of Nanchang University, Nanchang 330006, China
Many people are now suffering from unprecedented haze around the world [1], which has caused an adverse impact on human health and pollution on living environment [2]. According to the source classification, haze can be divided into three categories, large particulate matter (PM) (diameter in 11– 100 μm), coarse PM (2.5–10 μm) [3] and fine PM (≤ 2.5 μm, PM2.5) [4]. PM2.5 is the primary cause of damage to the respiratory and cardiovascular systems [5], including the irritation of respiratory tract [6], cough [7], dyspnea [8], the reduction of lung function [9], worsen asthma [10], chronic bronchitis [11], and arrhythmia [12]. In recent years, some effective techniques for air pollution were brought forward, such as static electricity [13], air ionization [14], activated carbon filtration [15], etc. However, these methods have disadvantages like high energy and economic consumption and tedious processes.
Aerogel, an air adsorbent material with characteristics of high specific surface area [16, 17], high porosity [18, 19] and ultra-low density [20-22], has vast potential in the field of materials science [23]. Herein, we peoduced black fungus aerogel, which is a noval material owned perfect adsorbability for PM2.5 and PM10. Moreover, after an in-situ modification of ZnO nanosheets, the black fungus aerogel has been appended other meaningful properties, which include broad-spectrum antibacterial activity and adsorption capacity for formaldehyde. This material represents an ideal air filter material, given that it is non-toxic, pollution-free and energy efficient.
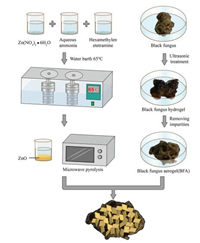
The research started with an interesting daily-life observation. Fig. 1 provided a schematic of the black fungus aerogels introduced in this work. The relative black fungus derived carbonaceous aerogels were then prepared by ultrasonic freeze-drying method. Exemplified with black fungus aerogel (BFA), we explored properties of each sides of the black gungus from two aspects: water contact angle measurements, and air pollutant adsorption abilities. Our journey commenced with the study on smooth and coarse surface of black fungus. The smooth and coarse surface of black fungus, BFA were shown in Fig. S1 (Supporting information). A distinct difference of the surface wettability occurred between the smooth and coarse surface of black fungus and the corresponding BFA.
|
Download:
|
| Fig. 1. Schematic of the fabrication process of black fungus aerogel (BFA) and BFA/ ZnO. | |
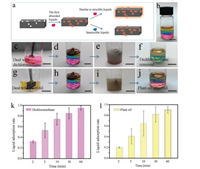
To further demonstrate the amphipathicity of BFA, we did the liquid selective absorption experiments. BFA had different adsorption rates for dichloromethane (DCM), plant oil and water. As presented in Fig. S2 (Supporting information), by comparing the absorption behavior, we found that BFA performed a high absorption capacity for all of the aforementioned liquids. Besides, the absorbability of BFA to DCM was better than to plant oil or water. Some unexpected and meaningful phenomena were uncovered in further experiments. For example, after treatment with DCM, BFA shows obvious hydrophobicity. In the meanwhile, it can selectively adsorb DCM. The same result could also be observed when the pre-treated BFA was used to absorb plant oil. So, we audaciously speculated that the pre-treated BFA has a certain ability which can absorb those liquids selectively. The mechanism is shown in Fig. 2a. The following presupposition could account for that phenomenon as well. The BFA would selectively absorb the similar liquids which were dissolvable in the original liquids, while the indissolvable liquids were repelled, suggesting that the first liquids played an important part in this process. To directly verify our hypothesis, a stratified liquid visualized system composed of plant oil, water and DCM (respectively stained with phosphor powder and fluorescent blue) was prepared (Fig. 2b). After shaking the stratified liquid system (Figs. 2e and i), the DCM and plant oil were absorbed (Figs. 2f and j) by different BFAs pretreated with DCM (Fig. 2c) and plant oil (Fig. 2g) respectively. As shown in Figs. 2k and l, the BFA endowed better pollutants absorption capacity. This surprising property can be used in the field of water pollutant treatment, for its precise pollutant adsorption characteristics.
|
Download:
|
| Fig. 2. The selective liquid absorption capacity of BFA. (a) Schematic of the liquid absorption mechanism. (b) A visual hierarchical liquid system consisting of plant oil, water and chloroform, respectively stained with phosphor powder and fluorescent blue. (c, g) The BFAs were pre-treated with dichloromethane and plant oil, respectively. (d, h) The BFAs were put into the system. (e, i) Shaking the liquid hierarchical systems. (f, j) The dichloromethane and plant oil were absorbed respectively. (The scale bar is 1 cm.) (k, l) The result of BFAs' liquid absorption rate. | |
The introduction of ZnO nanosheets endowed BFA with superior property. Scanning electron microscopy (SEM) images showed that both black fungus (Figs. 3a and b) and black fungus aerogel (BFA) (Figs. 3c and d) exhibited porous, interconnected, and well-organized 3D network structure, which are beneficial for filtering usage. The porous characteristics of BFA/ZnO were characterized by nitrogen adsorption and desorption experiments. The nitrogen adsorption–desorption isotherms and pore size distributions of BFA, ZnO and BFA/ZnO were shown in Figs. S3 and S4 (Supporting information). As shown in Figs. 3e and f, the ZnO nanosheets were observed on the surface of BFA. In addition, Fig. 3g and Fig. S5 (Supporting information) showed elemental mapping of BFA/ZnO. The neatly lamellar structures of ZnO nanosheets were densely impregnated on the surface of the aerogel (Fig. 3h and Fig. S6 in Supporting information), which matched well with the result of XRD (Fig. S7 in Supporting information).

|
Download:
|
| Fig. 3. SEM images of the smooth and coarse surface of (a, b) black fungus, (c, d) BFA and (e, f) BFA/ZnO. (g) Elemental mapping of BFA/ZnO. (h) SEM images of the ZnO nanosheets. | |
In addition, the BFA possessed high liquid absorption capacities. It was observed that both the black fungus and BFA had preferable hydrophilicity. From a micro prospective the coarse surface does not have compact arrangement of microstructure just as the smooth surface does. Many tiny gaps and cracks exist on the coarse surface, which was vulnerable to the water molecules. On the other hand, along with this compact microstructure, the smooth surface also possesses better adsorption efficiency for atmospheric pollutants, especially for the smaller sized PM2.5 particles. As summarized in Fig. 4a, the smooth surface of BFA had the best adsorption performance for PM2.5 and PM10. Hence, it was chosen in the subsequent study for adsorbing usage. The results of the above two experiments indicated that some natural plants with special biological microstructure, after the areogel related preparation process, could still maintain at least part of its microstructure derived properties. Notably, ZnO nanosheets exhibited even better adsorption properties for formaldehyde. And this intriguing discovery provided a good foundation for our subsequent research.

|
Download:
|
| Fig. 4. (a) The filtration rate of the smooth and coarse surface of BFA and BFA/ZnO tested with ambient particulate matter. (b) The inhibition zone area for Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) of BFA and BFA/ZnO. | |
An ideal adsorption agent should be predominantly antibacterial. To improve the antibacterial property, ZnO nanosheets were in situ decorated on BFA. To investigate the antibacterial activities of BFA and BFA/ZnO, we assessed the bacteriostatic action by E. coli and S. aureus respectively. The antibacterial activities of BFA and BFA/ZnO were then studied (Fig. 4b). Since the modification of ZnO nanosheets could improve the antibacterial activity of BFA/ZnO, a smooth surface group of BFA/ZnO was also added in this series of antibacterial tests. As expected, the smooth surface of BFA/ZnO possessed excellent antibacterial activity.
In conclusion, the preparation and applications of natural extracted aerogels on air pollution treatment [24] were investigated for the first time in this study. Two sides of black fungus aerogel (BFA) possessed different efficiency of filtering ambient particulate matter [25]. With the increasing amount of particulate matter in the atmosphere, people are suffering from environmental pollution seriously. Using crude black fungus as the sole carbon source, we have successfully prepared carbon aerogel with the merit of economize energy. Thanks to the advantages of high specific surface area and low density, this carbon aerogel has remarkable filtration effect on atmospheric aerosol. The antibacterial properties were further improved after the nano-modification [26]. We believe that the applications of these ultralight materials would be extended to other fields, such as biomedical dressing and bionic implants.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21461015, 31860263 to Xiaolei Wang; No. 91639106 to Hongbo Xin), Science Foundation of Jiangxi Provincial Department of Education (Nos. KJLD14010, 20153BCB23035, 20161ACB21002, 20165BCB19002 to Xiaolei Wang) and Nanchang University Seed Grant for Biomedicine.
Appendix A. Supplementary dataSupplementary material related to this article can befound, inthe online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.061.
| [1] |
R.C. Ho, M.W. Zhang, C.S. Ho, et al., BMC Psychiatry 14 (2014) 81. DOI:10.1186/1471-244X-14-81 |
| [2] |
M.C. Power, M.A. Kioumourtzoglou, J.E. Hart, et al., Br. Med. J. 350 (2015) h1111. |
| [3] |
M. Lippman, Crit. Rev. Toxicol. 44 (2014) 299-347. DOI:10.3109/10408444.2013.861796 |
| [4] |
R. Liang, B. Zhang, X. Zhao, et al., J. Hypertens. 32 (2014) 2130-2141. DOI:10.1097/HJH.0000000000000342 |
| [5] |
G. Polichetti, S. Cocco, A. Spinali, et al., Toxicology 261 (2009) 1-8. DOI:10.1016/j.tox.2009.04.035 |
| [6] |
F. Dominici, R.D. Peng, M.L. Bell, et al., Jama 295 (2006) 1127-1134. DOI:10.1001/jama.295.10.1127 |
| [7] |
M. Zheng, G.R. Cass, J.J. Schauer, et al., Environ. Sci. Technol. 36 (2002) 2361-2371. DOI:10.1021/es011275x |
| [8] |
X. Zeng, X. Xu, X. Zheng, et al., Environ. Pollut. 210 (2016) 346-353. DOI:10.1016/j.envpol.2016.01.025 |
| [9] |
W.J. Gauderman, G.F. Gilliland, H. Vora, et al., Crit. Care Med. 166 (2002) 76-84. DOI:10.1164/rccm.2111021 |
| [10] |
J.A. Gleason, L. Bielory, J.A. Fagliano, Environ. Res. 132 (2014) 421-429. DOI:10.1016/j.envres.2014.03.035 |
| [11] |
X. Hu, Y. Zhang, Z. Ding, et al., Atmos. Environ. 57 (2012) 146-152. DOI:10.1016/j.atmosenv.2012.04.056 |
| [12] |
R.B. Schlesinger, Inhalation Toxicol. 19 (2007) 811-832. DOI:10.1080/08958370701402382 |
| [13] |
P. Aarnio, T. Yli-Tuomi, A. Kousa, et al., Atmos. Environ. 39 (2005) 5059-5066. DOI:10.1016/j.atmosenv.2005.05.012 |
| [14] |
M.F. Khan, Y. Shirasuna, K. Hirano, et al., Atmos. Res. 96 (2010) 159-172. DOI:10.1016/j.atmosres.2009.12.009 |
| [15] |
H.L. Clack, Environ. Sci. Technol. 40 (2006) 3617-3622. DOI:10.1021/es050246+ |
| [16] |
Y. Zhang, J.Y. Zhu, H.B. Ren, et al., Chin. Chem. Lett. 28 (2017) 935-942. DOI:10.1016/j.cclet.2017.01.023 |
| [17] |
X. Lin, H. Lou, W. Lu, et al., Chin. Chem. Lett. 29 (2018) 633-636. DOI:10.1016/j.cclet.2017.11.024 |
| [18] |
H. Zhuo, Y. Hu, X. Tong, et al., Adv. Mater. 30 (2018) 1706705-1706714. DOI:10.1002/adma.201706705 |
| [19] |
Y. Tang, K.L. Yeo, Y. Chen, et al., J. Mater. Chem. A 1 (2013) 6723-6726. DOI:10.1039/c3ta10969k |
| [20] |
L. Dong, C. Liu, F. Yu, et al., J. Mater. Chem. B 5 (2017) 6217-6220. DOI:10.1039/C7TB01377A |
| [21] |
G. Yang, X. Liu, V. Lipik, et al., J. Mater. Sci. 53 (2018) 9463-9472. DOI:10.1007/s10853-018-2244-1 |
| [22] |
D. Lin, P.Y. Yuen, Y. Liu, et al., Adv. Mater. 30 (2018) 1802661-1802669. DOI:10.1002/adma.201802661 |
| [23] |
Q.J. Luo, Y.X. Li, M.Q. Zhang, et al., Chin. Chem. Lett. 28 (2017) 345-349. DOI:10.1016/j.cclet.2016.10.024 |
| [24] |
Y. Zhang, P. Gao, Q. Yue, et al., Chin. Chem. Lett. 29 (2018) 1661-1665. DOI:10.1016/j.cclet.2018.06.023 |
| [25] |
Y.X. Zhang, Z.H. Yang, Q.X. Zhang, et al., Chin. Chem. Lett. 26 (2015) 157-159. DOI:10.1016/j.cclet.2014.08.004 |
| [26] |
R.J. Li, X.J. Kou, H. Geng, et al., Chin. Chem. Lett. 25 (2014) 663-666. DOI:10.1016/j.cclet.2014.03.032 |
 2019, Vol. 30
2019, Vol. 30 

