b State Key Laboratory of Food Science and Technology, Nanchang University, Nanchang 330047, China
Glucose is a key intermediate product in metabolic processes, and its concentration at each stage in the metabolic process relates directly to human health. At the same time, it is very necessary to monitor the concentration of glucose in the body fluids in the process of relevant disease diagnosis, treatment and control. Diabetes is a metabolic disease characterised by high blood sugar levels for a long time. High blood sugar is a condition in which an excessive amount of glucose circulates in the blood plasma. This is generally a blood sugar level higher than 11.1 mol/L, but symptoms may not start to become noticeable until even higher values such as 15–20 mol/L [1-4]. When left untreated, it can cause serious health problems, such as stroke, cardiovascular disease, chronic nephropathy, foot ulcers and damage to the eyes, which will significantly increase morbidity and mortality among diabetics and bring about severe economic burden for individuals and countries [5, 6]. To detect blood sugar level accurately and conveniently, researchers have made tremendous efforts efforts to develop glucose sensors with high sensitivity, good selectivity, easy operation and inexpensiveness. Basing on electrochemical sensor techniques, a number of glucose enzyme biosensors have been studied and developed [7-10]. However, enzymatic activity is susceptible to external environment, such as temperature, atmospheric humidity, pH value and toxic chemicals, etc. [11, 12]. So the requirements for experimental conditions based on enzyme biosensors are very strict.
As expected, the non-enzymatic electrochemical sensors can overcome the disadvantage of enzyme-based sensors, but there are still some problems in practical application, such as low sensitivity and poor selectivity.
In general, nano-composites based on transition metals or their oxides exert excellent electrocatalytic performance for glucose [13-16]. Cobalt tetroxide (Co3O4) is an important transition metal oxide with a normal spinel crystal structure, and has outstanding properties such as low cost, good chemical stability, excellent semi-conductivity and good biocompatibility, which has been extensively applied in photocatalysis [17], supercapacitors [18-20], lithium-ion batteries [21-23] and electrochemical sensors [24]. However, these Co3O4-based catalysts usually suffer from the poor electrical conductivity, short active site density. Carbon nanotubes (CNTs) are allotropes of carbon with a cylindrical nanostructure with extraordinary thermal conductivity, mechanical, and electrical properties, which are valuable for nanotechnology, electronics, optics and other fields of materials science and technology. In addition, carbon nanotubes find applications as additives to various structural materials [25, 26]. Wang et al. [27] reported that Co3O4@MWCNT nanocable was used as a cathode material for supercapacitors and showed excellent electrochemical performance.
The aims of this study were as follows: (1) Prepare stable nanocomposite of Co3O4-MCNT by a solvothermal method; (2) Investigate electrocatalytic performance of glucose at the Co3O4- MCNT/GCE; (3) Develop an excellent quantitative method for detection of glucose in Co3O4-MCNT/GCE with the use of amperometric (i–t) technique.
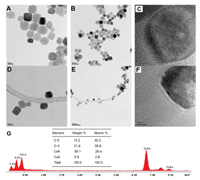
The morphology and structural features of Co3O4 and Co3O4- MCNT were characterized by TEM. From Figs. 1A–C, it is very obviously that prepared Co3O4 nanoparticles have cube-like structure, and their average diameter was approximately 15 nm. From Figs. 1D–F, it can be observed that the Co3O4 nanoparticles are uniformly distributed around MNCTs.
|
Download:
|
| Fig. 1. TEM images of Co3O4 (A, B and C) and Co3O4-MCNT (D, E and F); EDS of Co3O4-MCNT (G). | |
EDS test was performed to evaluate chemical composition of Co3O4-MCNT (Fig. 1G) and affirm the existence of Co, O, C and Cu. Furthermore, according to the results, the molar ratio of Co and O element is about 3:4.
Electrochemical response to glucose (50.0 mmol/L) at the Co3O4-MCNT/GCE was evaluated in different concentration of NaOH solution at the applied potential from 0.2 V to 0.6 V. The experimental results showed that maximum current change of glucose was obtained at the potential of +0.50 V in 0.1 mol/L NaOH, which was consequently selected for subsequent amperometric i-t measurements.
Cyclic voltammetric (CV), electrochemical impedance spectroscopic (EIS) and chronocoulometric measurements were all performed on CHI660A electrochemical workstation, with a three-electrode electrochemical cell, containing 5 ×10-3 mol/L [Fe(CN)6]3-/4- (1:1) and 0.1 mol/L KCl aqueous solution as electrolyte.
From CV experimental results (Fig. 2A), a pair of well defined redox peak was observed at the bare GCE and the change of the redox peak potential (∆E) was 0.102 V (curve a, Fig. 2A). After modification of Co3O4 nano-materials, the current value decreased slightly with the ∆E increased to 0.196 V (curve b, Fig. 2A). Then, when Co3O4-MCNT/GCE was tested, the oxidation and reduction peak current significantly increased with the ∆E decreased to 0.172 V (curve c, Fig. 2A) due to the excellent conductivity of MCNT.

|
Download:
|
| Fig. 2. (A) Cyclic voltammogram (CV), (B) ectrochemical impedance spectroscopy (EIS) and (C) chronocoulometric plots from: (a) GCE, (b) Co3O4/GCE, and (c) Co3O4-MCNT/GCE in 5 ×10-3 mol/L [Fe(CN)6]3-/4- (1:1) solution containing 0.1 mol/L KCl. | |
Under the same experimental conditions, EIS experiments (Fig. 2B) gave a similar conclusion obtained from the CV work above, i.e., the response profile for the bare GCE (curve a, Fig. 2B) was effectively a small semi-circle. When the Co3O4 were covered on the surface of the GCE and a larger semi-circle was observed. This indicated the presence of a relatively high electron transfer resistance and suggested that the Co3O4/GCE had poor conductivity (curve b, Fig. 2B). However, when the Co3O4-MCNT/GCE (curve c, Fig. 2B) was tested, the impedance curve of the modified electrode was further reduced as compared to that from the Co3O4/ GCE. Thus, this observation suggested that the conductivity of MCNT is good.
Chronocoulometric results were used to compare the specific surface area of the electrode with the use of Eq. (1) [28]:
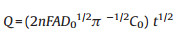
|
(1) |
where Q is the absolute value of the reduction charge, n is the number of electrons transferred, F is Faraday constant, A is the apparent electrode area, t is time, and D0 and C0 is the diffusion coefficient and the bulk concentration of the oxidized form of the hexacyanoferrate(III) complex, respectively.
The apparent electrode area, A, may be estimated from the slope of the Q versus t1/2 plot (Fig. 2C). Thus, the order of the slope values was: Co3O4-MCNT/GCE (c) > Co3O4/GCE (b) > GCE (a), i.e., Co3O4- MCNT/GCE has the largest A value, and thus, this modified electrode showed the best electrochemical activity.
Electrochemical properties of glucose in Co3O4-MCNT/GCE were investigated in 0.1 mol/L NaOH solution in the range of 0.02– 0.30 V/s. As shown in Fig. S1A (Supporting information), two pairs of redox peaks can be observed at the different potentials, which can be due to redox reactions of Co3O4 nanoparticles under alkaline conditions corresponding to the following equations [29, 30]:

|
(2) |
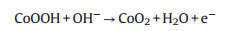
|
(3) |
Redox peak currents were directly proportional to the potential scan rate (v) in the range of 0.02–0.30 V/s (Fig. S1B in Supporting information), which indicated that electrochemical process of Co3O4-MCNT/GCE was mainly controlled by adsorption [28].
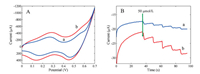
Fig. 3A shows CVs of Co3O4-MCNT/GCE in 0.1 mol/L NaOH without (curve a) and with (curve b) 50.0 mmol/L glucose. The results showed that redox peak currents increased obviously after addition of 50.0 mmol/L glucose.
|
Download:
|
| Fig. 3. (A) CVs of Co3O4-MCNT in (a) absence and (b) presence of 50 mmol/L glucose in 0.1 mol/L NaOH solution at 0.10 V/s; (B) Amperometric response recorded for Co3O4/ GCE (a) and Co3O4-MCNT/GCE (b). | |
To investigate electrochemical performance of differently modified electrodes, amperometric (i–t) curves of modified electrodes were recordedwith continuous stirring and successive step changes in glucose at +0.50 V in 0.1 mol/L NaOH. As shown in Fig. 3B, a much larger catalytic current was observed at the Co3O4-MCNT/GCE. This significant improvement can be attributed to synergistic catalytic effects of Co3O4 and MCNT. From Fig. 2C, when Co3O4 and MCNT were combined, the specific surface area of the Co3O4-MCNT/GCE greatly increased. Similar to previous studies [29, 30], electrochemical reactions for glucose were possibly the following:
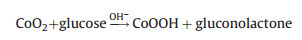
|
(4) |
Under alkaline condition, Co3O4 can easily transform into CoOOH under high potential conditions (+0.5 V), CoOOH can further be oxidized to generate a large number of CoO2 intermediate products, which can oxidize glucose to gluconolactone. As to the reversible redox reactions, the recover time for completing a cycle is 14 s. According to reactions (3) and (4), this electorde reaction mechanism can be as follows:

|
(5) |
Quantitative analysis was performed with the use of amperometric (i–t) technique under optimal experimental conditions with glucose concentration from 1.0 mmol/L to 545 mmol/L (Fig. 4A). The results showed that the plot of current difference versus concentration of glucose was linear in the range of 1.0– 122 mmol/L, with a linear correlation coefficient (R2) of 0.9983 and limit of detection (LOD) of 0.28 mmol/L (Fig. 4B). Corresponding sensitivity of glucose sensor measured 2550 mA L mmol-1 cm-2. For comparative purposes, Table S1 (Supporting information) lists the analytical range, sensitivity and LOD values based on Co3O4- related electrode materials.

|
Download:
|
| Fig. 4. Amperometric response (A and B) of Co3O4-MCNT/GCE after successive additions of glucose in 0.1 mol/L NaOH at +0.50 V; (C) Amperometric response of the Co3O4- MCNT/GCE to different chemicals in 0.1 mol/L NaOH at +0.50 V. Inset: the calibration plot. | |
Anti-interference capacity of the Co3O4-MCNT/GCE was investigated under the optimum test conditions described above, and different common interfering substances were used, e.g., dopamine (DA), ascorbic acid (AA), K+, Na+, Mg2+ and Ca2+. Current versus time (i–t) plot (Fig. 4C) indicate that these substances did not affect the determination of glucose.
To estimate reproducibility and stability of sensor, Co3O4-MCNT/GCE was constructed separately three times using the same GCE. The same concentration of glucose solution (20.0mmol/L) was used as test sample, and the current changes (∆I) of the amperometric (i–t) technique were recorded. Average relative standard deviation (% RSD) was evaluated as 4.78% from the three measurement results, which indicated satisfactory reproducibility of the sensor. Furthermore, the modified electrode was stored at 4 ℃ for two weeks, and current intensity for glucose decreased by only 6.36% compared with the original response on glucose sample of 20.0 mmol/L. Results suggest good stability of Co3O4-MCNT/GCE sensor.
To illustrate the feasibility of the sensor in practical analysis and application, the proposed method was applied to analyse the content of glucose in human serum [31]. To keep the test conditions constant, the human serum samples were 50-fold diluted with 0.1 mol/L NaOH before the measurements, and above preprocessed human serum samples (1.0 mL) were transferred to an electrochemical cell and diluted to 10.0 mL with 0.1 mol/L NaOH. The Co3O4-MCNT/GCE, SCE and platinum wire were immersed the electrochemical cell and performed with the use of amperometric (i–t) technique under optimal experimental conditions. The recovery experiments were carried out by spiking with standard glucose solutions (2 mmol/L). From Table 1, it can be seen that the sensor gives good recoveries (96.1%–104.8%), indicating that the proposed analytical method was satisfactory for the analysis of glucose in human serum samples and may have a potential for practical application.
|
|
Table 1 Results of quantitative analyses of glucose in human serum (n = 3). |
In summary, a new non-enzymatic electrochemical glucose sensor, Co3O4-MCNT/GCE, was successfully constructed to analyse glucose at low concentration levels. The sensor achieved highly sensitive and stable glucose detection, which may be because the Co3O4-MCNT/GCE has a large specific surface area, effectively supporting a large number of electroactive substances, and thus significantly enhancing proton and electron transfer. Moreover, this result maybe be related to synergistic catalytic effects of Co3O4 and MCNT. To investigate practical application of this sensor, it was used to monitor trace amounts of glucose in human serum samples. Significantly, results also showed that the proposed method may feature potential practical applications.
AcknowledgmentThe authors gratefully acknowledge the financial support of this study by the National Natural Science Foundation of China (NSFC, No. 31860468).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.04.009.
| [1] |
G. Yong, Y.G. Zhang, J.Y. Ying, Angew. Chem. Int. Ed. 47 (2008) 9345-9348. DOI:10.1002/anie.200803207 |
| [2] |
H.F. Bunn, P.J. Higgins, Science 231 (1981) 222-224. |
| [3] |
J. Xu, C.L. Bartolome, C.S. Low, et al., Nature 556 (2018) 505. DOI:10.1038/s41586-018-0049-7 |
| [4] |
Y.F. Xiao, H. Sung, J.Z. Du, J. Am. Chem. Soc. 139 (2017) 7640-7647. DOI:10.1021/jacs.7b03219 |
| [5] |
S.Z. Swidan, P.A. Montgomery, Pharmacotherapy 18 (1998) 961-972. |
| [6] |
Whaley-Connell A., J.R. Sowers, Diabetes 63 (2014) 45-47. DOI:10.2337/db13-1497 |
| [7] |
M. Gu, J.W. Wang, Y.F. Tu, J.W. Di, Sens. Actuators B:Chem. 148 (2010) 486-491. DOI:10.1016/j.snb.2010.05.057 |
| [8] |
L. Mauko, B. Ogorevc, B. Pihlar, Electroanalysis 21 (2009) 2535-2541. DOI:10.1002/elan.v21:23 |
| [9] |
J.H. Kim, S.A. Jun, Y. Kwon, et al., Bioelectrochemistry 101 (2015) 114-119. DOI:10.1016/j.bioelechem.2014.08.017 |
| [10] |
M. Xiong, B. Gu, J.D. Zhang, et al., Biosen. Bioelectron. 50 (2013) 229-234. DOI:10.1016/j.bios.2013.06.030 |
| [11] |
R. Wilson, A.P.F. Turner, Biosens. Bioelectron. 7 (1992) 165-185. DOI:10.1016/0956-5663(92)87013-F |
| [12] |
C.M. Wong, K.H. Wong, X.D. Chen, Appl. Microbiol. Biotechnol. 78 (2008) 927-938. DOI:10.1007/s00253-008-1407-4 |
| [13] |
Y. Liu, C.M. Deng, L. Tang, et al., J. Am. Chem. Soc. 133 (2011) 660-663. DOI:10.1021/ja107086y |
| [14] |
S.A. Zaidi, J.H. Shin, Talanta 149 (2016) 30-42. DOI:10.1016/j.talanta.2015.11.033 |
| [15] |
Q.Y. Liu, P.P. Chen, Z. Xu, et al., Sens. Actuators B:Chem. 251 (2017) 339-348. DOI:10.1016/j.snb.2017.05.069 |
| [16] |
Y. Su, H. Guo, Z.S. Wang, et al., Sens. Actuators B:Chem. 255 (2018) 2510-2519. DOI:10.1016/j.snb.2017.09.056 |
| [17] |
A.C. Pradhan, T. Uyar, ACS Appl. Mater. Interfaces 41 (2017) 35757-35774. |
| [18] |
H. Pang, X.R. Li, Q.X. Zhao, et al., Nano Energy 35 (2017) 138-145. DOI:10.1016/j.nanoen.2017.02.044 |
| [19] |
M. Huang, Y.X. Zhang, F. Li, et al., J. Power Sources 252 (2014) 98-106. DOI:10.1016/j.jpowsour.2013.12.030 |
| [20] |
J. Xu, Q.F. Wang, X.W. Wang, et al., ACS Nano 7 (2013) 5453-5462. DOI:10.1021/nn401450s |
| [21] |
D. Gu, W. Li, F. Wang, et al., Agnew. Chem. Int. Ed. 54 (2015) 7060-7064. DOI:10.1002/anie.201501475 |
| [22] |
X. Li, X.D. Tian, T. Yang, Y. Song, Z.J. Liu, Chem. -Eur. J. 24 (2018) 14477-14483. DOI:10.1002/chem.201802715 |
| [23] |
X.W. Wang, F.X. Wang, L.Y. Wang, et al., Adv. Mater. 28 (2016) 4904-4911. DOI:10.1002/adma.v28.24 |
| [24] |
H.Y. Xu, C.K. Xia, S.Y. Wang, et al., Sens. Actuators B:Chem. 267 (2018) 93-103. DOI:10.1016/j.snb.2018.04.023 |
| [25] |
V. Datsyuk, M. Kalyva, K. Papagelis, et al., Carbon 46 (2018) 833-840. |
| [26] |
G.C. Yu, M. Xue, Z.B. Zhang, et al., J. Am. Chem. Soc. 134 (2012) 13248-13251. DOI:10.1021/ja306399f |
| [27] |
X.W. Wang, M.X. Li, Z. Chang, et al., ACS Appl. Mater. Interfaces 7 (2015) 2280-2285. DOI:10.1021/am5062272 |
| [28] |
X.Y. Lin, Y.F. Wang, W.H. He, Y.N. Ni, S. Kokot, RSC Adv. 7 (2017) 55460-55467. |
| [29] |
L. Wang, Q.Y. Zhang, S.L. Chen, et al., Anal. Chem. 86 (2014) 1414-1421. DOI:10.1021/ac401563m |
| [30] |
K.E. Toghill, R.G. Compton, Int. J. Electrochem. Sci. 5 (2010) 1246-1301. |
| [31] |
X. Zhong, R. Yuan, Y.Q. Chai, Chem. Commun. 48 (2012) 597-599. DOI:10.1039/C1CC16081H |
 2019, Vol. 30
2019, Vol. 30