b Key Laboratory of Hunan Province for Water Environment and Agriculture Product Safety, Changsha 410083, China
Hypochlorous acid (HClO) is one of the most common reactive oxygen species (ROS) [1-3]. Endogenous HClO plays important roles in physiological and pathological processes [4-7]. For example, hypochlorous acid can resist inflammation and regulate cellular apoptosis due to its antibacterial ability [8-10]. However, overexpressed hypochlorous acid could induce many diseases, such as osteoarthritis, cardiovascular diseases, lung injury atherosclerosis, and cancers [11-13]. As a consequence, the qualitative and quantitative detection of hypochlorous acid with a rapid response is very meaningful for medical diagnosis and treatment [14].
In the past decade, many conventional methodologies, including colorimetry and electrochemical analysis, mass spectrometry, HPLC analysis and bioanalytical methods etc. have been developed to determine hypochlorous acid [15-18]. While these abovementioned technologies are useful for the detection of hypochlorous acid in vitro, neither of them is able to sense hypochlorous acid in vivo, especially in living organisms. Fluorescent chemosensors emerged as a reliable tool for the detection of hypochlorous acid in vitro and in vivo with high temporal-spatial, good selectivity and sensitivity. As a result, many fluorescent probes were developed for detecting and imaging hypochlorous acid recently [19-24]. Compared with intensity-based fluorescent probes, ratiometric ones are more desirable because they can reduce the interference from the background, minimize influence of the concentration of probe and other environmental factors through self-calibration with two separated fluorescence signals. Moreover, long-wavelength emissions and large Stokes shift are beneficial properties for fluorescent probes because long-wavelenght photons can deeply penetrate tissues and large Stokes shift can avoid the selfabsorption issue [25, 26]. Therefore, ratiometric fluorescence probes with long-wavelength emission and large Stokes shift are more desirable for imaging hypochlorous acid.
Naphthalimide-based fluorescent probes have been developed due to their advantageous optical properties and easy preparation [27-31]. In this work, we developed a red-emitting fluorescent probe, NClO, for the detection of hypochlorous acid (Scheme 1) with a large Stokes shift based on a naphthalimide derivative. The double bond between naphthalimide and methylated pyridine in probe NClO could be oxidized by hypochlorous acid, which would induce a blue shift of emission to display a ratiometric fluorescence change (Scheme 2). The fluorescence behavior of this probe in response to hypochlorous acid in the solution and in living cells suggested that probe NClO could detect hypochlorous acid with high sensitivity and selectivity in vitro and in vivo. The synthetic route of NClO was illustrated in Scheme 1.
|
Download:
|
| Scheme 1. Synthetic route of probe NClO. | |

|
Download:
|
| Scheme 2. Proposed sensing process of probe NClO toward HClO. | |
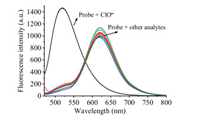
Spectral measurements of probe NClO towards HClO subsequently were carried out in PBS buffer (10.0 mmol/L, pH 7.4, containing 10% acetonitrile) at room temperature. As shown in Fig. 1, probe NClO displayed a red emission with a maximum at 615 nm. When the solution of probe NClO was treated with excessive HClO, green fluorescence signals with λem, max = 520 nm were observed. However, negligible fluorescence changes were seen when the solution of probe NClO was in the presence of other ROS/RNS, including ·OH, 1O2, H2O2, KO2, NO, t-BuO·, ONOO–, ROO· and TBHP. Therefore, it could be concluded that probe NClO exhibited a good selectivity for HClO.
|
Download:
|
| Fig. 1. Fluorescence response of probe NClO (5.0 μmol/L) to various ROS/RNS (110 μmol/L) including HClO, ·OH, 1O2, H2O2, KO2, NO, t-BuO·, ONOO-, ROO· and TBHP in CH3CN-PBS buffer (1/9 (v/v), 10.0 mmol/L, pH 7.4). | |
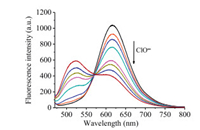
Next, we carried out concentration-dependent absorption and emission (Figs. S1 and S2 in Supporting information) experiments on probe NClO with different amount of HClO. As shown in Fig. 2, the incremental addition of HClO resulted in gradual decreases in fluorescence intensity at 615 nm and a new progressivelyincreased green emission at 520 nm. And the ratio of fluorescence intensity at 520 nm and 615 nm (I520/I615) was linear to the concentration of HClO in a range of 0.0–30.0 μmol/L (Fig. S3 in Supporting information). The detection limit for HClO was as low as 19 nmol/L based on S/N (signal to noise) = 3. These results clearly indicated that the probe NClO had a high sensitivity to HClO.
|
Download:
|
| Fig. 2. Fluorescence spectral of probe NClO (5.0 μmol/L) with HClO (0.0– 30.0 μmol/L) in CH3CN-PBS buffer (1/9 (v/v), 10.0 mmol/L, pH 7.4). Data obtained after 10 min of incubation. λex = 450 nm. | |
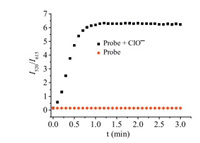
Time-dependent fluorescence experiment on probe NClO (5.0 μmol/L) in the presence of HClO (22 equiv.) showed that an equilibrium of the fluorescence intensity ratio (I520/I615) was quickly reached (within 1 min) (Fig. 3). In contrast, there was no fluorescence change in the solution of probe NClO when HClO was absent. The kinetic studies revealed that probe NClO was stable and could act as a fluorescent probe for rapid response to HClO.
|
Download:
|
| Fig. 3. Ratio of fluorescence intensity of NClO (5.0 μmol/L) at 520 nm and 615 nm in the absence/presence of ClO- (110 μmol/L) as a function of time. | |
To verify whether probe NClO can be conducted in physiological condition, the performance of probe NClO was assessed by investigating its fluorescence behavior in a pH range of 4.0–9.0. As shown in Figs. S4 and S5 (Supporting information), it was seen that the ratio of I520/I615 was little and hardly changed when probe NClO was in the absence of HClO, indicating probe NClO had a good stability between pH 4.0–9.0. In the presence of HClO, the ratio of I520/I615 remarkably increased and kept stable in the physiological pH range. These results indicated that probe NClO could be used as a ratiometric probe for HClO under physiological condition.
The sensing mechanism of probe NClO toward HClO was investigated by mass spectral analysis. As shown in Fig. S9 (Supporting information), a peak at m/z 296.0902 was displayed in the mass spectra of the solution of probe NClO with HClO, which was nearly equal to the exact molecular weight of compound 1 ([M-H]- = 296.0928). This experimental result strongly confirmed our proposed mechanism in Scheme 2.
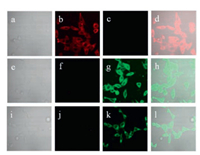
Finally, the application of probe NClO was performed in the detection of HClO in living cells. Before conducting cell imaging experiments, cytotoxic assays for probe NClO in HeLa cells were performed. And 96% of cell viability were obtained when cells were treated with 5.0 μmol/L of probe NClO for 24 h, indicating probe NClO was non-toxic (Fig. S10 in Supporting information). When cells were incubated with probe NClO (5.0 μmol/L) for 30 min at 37 ℃, strong fluorescence signals in red channel and no fluorescence signals in green channel were observed (Figs. 4b and c). In contrast, the incubation of NClO-pretreated cells with HClO resulted in a decrease of fluorescence in red channel and strong fluorescence in green channel (Figs. 4f and g). Next, phorbol-12- myristate-13-acetate (PMA) is used to stimulate cells to produce endogenous HClO for the imaging of endogenous HClO [26, 32]. When cells were treated with PMA (1 μg/mL) for 2 h and then incubated with probe NClO (5.0 μmol/L) for another 30 min at 37 ℃, only green fluorescence was seen from inside cells (Figs. 4j and k). These cell imaging results implied that probe NClO could penetrate cell membranes and displayed a ratiometric fluorescence change in detection HClO in living cells.
|
Download:
|
| Fig. 4. Cell images of HeLa cells. Top row: Cells incubated with probe NClO (5.0 μmol/L) for 30 min at 37 ℃. Middle row: Cells pretreated with probe NClO (5.0 μmol/L) for 30 min at 37 ℃ and then incubated with HClO (110 μmol/L) for another 30 min. Bottom row: Cells pretreated with PMA (1 μg/mL) for 2 h and then incubated with probe NClO (5.0 μmol/L) for 30 min at 37 ℃. (a, e, i) bright-field; (b, f, j) fluorescence from red channel; (c, g, k) fluorescence from green channel; (d, h, l) merged. Cells were magnified 40 times. | |
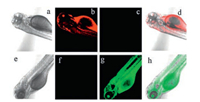
Furthermore, probe NClO also had been applied to image in zebra fish (Figs. 5). When zebra fish was fed with probe NClO (5.0 μmol/L), only bright red fluorescence was seen (Figs. 5b and c). Contrarily, only green fluorescence displayed when NClO-pretreated zebra fish was further incubated with HClO (Figs. 5f and g).
|
Download:
|
| Fig. 5. Images of zebra fish. Top row: Zebra fish incubated with probe NClO (5.0 μmol/L). Bottom row: Zebra fish treated with probe NClO (5.0 μmol/L) and subsequently incubated with HClO (110 μmol/L). (a, e) bright-field; (b, f) fluorescence from red channel; (c, g) fluorescence from green channel; (d, h) merged. | |
In conclusion, we developed a fluorescent probe NClO for the qualitative and quantitative detection of HClO with high sensitivity and selectivity in a ratiometric manner. This probe exhibited a quick response towards HClO with a low detection limit (19 nmol/L). It is noteworthy that probe NClO could image exogenous and endogenous HClO in living cells and zebra fish with good performance.
AcknowledgmentsThis study was supported by the National Natural Science Foundation of China (No. U1608222) and the State Key Laboratory of Fine Chemicals (No. KF1606). We acknowledge the financial support from Fundamental Research Funds for the Central Universities of Central South University (No. 2018zzts109).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.02.008.
| [1] |
M.R. Detty, P.B. Merkel, R. Hilf, S.L. Gibson, S.K. Powers, J. Med. Chem. 33 (1990) 1108-1116. DOI:10.1021/jm00166a005 |
| [2] |
D.I. Pattison, M.J. Davies, Chem. Res. Toxicol. 14 (2001) 1453-1464. DOI:10.1021/tx0155451 |
| [3] |
S. Drevet, G. Gavazzi, L. Grange, C. Dupuy, B. Lardy, Exp. Gerontol. 111 (2018) 107-117. DOI:10.1016/j.exger.2018.07.007 |
| [4] |
X. Chen, F. Wang, J.Y. Hyun, et al., Chem. Soc. Rev. 45 (2016) 2976-3016. DOI:10.1039/C6CS00192K |
| [5] |
X. Liu, Z. Tang, B. Song, H. Ma, J. Yuan, J. Mater. Chem. B 5 (2017) 2849-2855. DOI:10.1039/C6TB02991D |
| [6] |
H. Ma, B. Song, Y. Wang, et al., Chem. Sci. 8 (2017) 150-159. DOI:10.1039/C6SC02243J |
| [7] |
C. Yin, H. Zhu, C. Xie, et al., Adv. Funct. Mater. 27 (2017) 1700493. DOI:10.1002/adfm.v27.23 |
| [8] |
P. Nagy, K. Lemma, M.T. Ashby, J. Org. Chem. 72 (2007) 8838-8846. DOI:10.1021/jo701813f |
| [9] |
L. Qiao, H. Nie, Y. Wu, et al., J. Mater. Chem. B 5 (2017) 525-530. DOI:10.1039/C6TB02774A |
| [10] |
Q. Xu, C.H. Heo, G. Kim, et al., Angew. Chem. Int. Ed. Engl. 54 (2015) 4890-4894. DOI:10.1002/anie.201500537 |
| [11] |
Q.S. Lin, Y.L. Huang, X.X. Fan, et al., Talanta 170 (2017) 496-501. DOI:10.1016/j.talanta.2017.04.024 |
| [12] |
M. Ren, J. Nie, B. Deng, et al., N. J. Chem. 41 (2017) 5259-5262. DOI:10.1039/C7NJ00949F |
| [13] |
Q. Yuan, Z.M. Zhao, Y.R. Zhang, et al., Sens. Actuators B-Chem. 247 (2017) 736-741. DOI:10.1016/j.snb.2017.03.049 |
| [14] |
Y. Dai, S. Cheng, Z. Wang, et al., ACS Nano 12 (2018) 455-463. DOI:10.1021/acsnano.7b06852 |
| [15] |
J.D. García-Espinoza, P. Mijaylova-Nacheva, M. Avilés-Flores, Chemosphere 192 (2018) 142-151. DOI:10.1016/j.chemosphere.2017.10.147 |
| [16] |
J. Kalmár, M. Szabó, N. Simic, I. Fábián, Dalton Trans. 47 (2018) 3831-3840. DOI:10.1039/C8DT00120K |
| [17] |
R. Zhang, B. Song, J. Yuan, TrAC-Trends Anal. Chem. 99 (2018) 1-33. DOI:10.1016/j.trac.2017.11.015 |
| [18] |
B. Zhu, Y. Xu, W. Liu, et al., Sens. Actuators B-Chem. 191 (2014) 473-478. DOI:10.1016/j.snb.2013.10.057 |
| [19] |
B. Zhang, X. Yang, R. Zhang, et al., Anal. Chem. 89 (2017) 10384-10390. DOI:10.1021/acs.analchem.7b02361 |
| [20] |
P. Ning, W. Wang, M. Chen, Y. Feng, X. Meng, Chin. Chem. Lett. 28 (2017) 1943-1951. DOI:10.1016/j.cclet.2017.09.026 |
| [21] |
Y. Chen, T. Wei, Z. Zhang, et al., Chin. Chem. Lett. 28 (2017) 1957-1960. DOI:10.1016/j.cclet.2017.05.010 |
| [22] |
J. Li, X. Yang, D. Zhang, et al., Sens. Actuators B-Chem. 265 (2018) 84-90. DOI:10.1016/j.snb.2018.03.027 |
| [23] |
S.J. Li, D.Y. Zhou, Y.F. Li, et al., Talanta 188 (2018) 691-700. DOI:10.1016/j.talanta.2018.06.046 |
| [24] |
H. Tong, Y. Zhang, S. Ma, et al., Chin. Chem. Lett. 29 (2018) 139-142. DOI:10.1016/j.cclet.2017.07.007 |
| [25] |
B. Zhu, L. Wu, M. Zhang, et al., Biosens. Bioelectron. 107 (2018) 218-223. DOI:10.1016/j.bios.2018.02.023 |
| [26] |
Y. Gao, Y. Pan, Y. He, H. Chen, V.N. Nemykin, Sens. Actuators B-Chem. 269 (2018) 151-157. DOI:10.1016/j.snb.2018.04.135 |
| [27] |
X. Zhang, B. Wang, Y. Xiao, C. Wang, L. He, Analyst 143 (2018) 4180-4188. DOI:10.1039/C8AN00905H |
| [28] |
F. Yang, C. Wang, L. Wang, et al., Chin. Chem. Lett. 28 (2017) 2019-2022. DOI:10.1016/j.cclet.2017.07.030 |
| [29] |
H. Bian, X. Song, N. Li, H. Man, Y. Xiao, J. Mater. Chem. B 6 (2018) 1699-1705. DOI:10.1039/C7TB03279J |
| [30] |
C. Wang, X. Song, Y. Xiao, ChemBioChem 18 (2017) 1762-1769. DOI:10.1002/cbic.201700161 |
| [31] |
Y. Dai, B.K. Lv, X.F. Zhang, Y. Xiao, Chin. Chem. Lett. 25 (2014) 1001-1005. DOI:10.1016/j.cclet.2014.05.020 |
| [32] |
M.B. Iversen, R.H. Gottfredsen, U.G. Larsen, et al., Free Radic. Biol. Med. 97 (2016) 478-488. DOI:10.1016/j.freeradbiomed.2016.07.004 |
 2019, Vol. 30
2019, Vol. 30 

