Fluorescent imaging is a powerful technique in monitoring biological analytes and processes in living systems [1]. The developed fluorescent probes have made great contribution to the significant advances in cell biology and medical diagnostic imaging [2]. Near infrared (NIR) fluorescent probes are particularly favorable due to the distinct advantages of NIR light including deep tissue penetration, low background noise and minimum photodamage to biological samples [3]. However, the construction of small-molecule based NIR fluorescent probes is highly challenging and vigorously pursued.
Rhodamine derivatives have attracted extensive attention because of their high quantum yield, large extinction coefficients and good biocompatibility [4]. But the absorption and emission spectra of the rhodamine derivatives are not in the near infrared region, which is unfavorable for bioimaging in vivo. Therefore, in 2008, Qian reported the first Si-substituted xanthene derivatives as far-red to NIR fluorescent dyes [5]. Afterwards, Nagano extended this concept and developed various Si-rhodamines based on the Sixanthene platform [6-10]. Although the family of Si-rhodamine derivatives has been widely employed for functional fluorescent probes, there is still much room for improvement. For example, the developed Si-substituted xanthene derivatives usually have poor water solubility and are hardly modifiable, both of which will greatly hinder their further biological applications. Therefore, construction of novel water-soluble and easily modified skeleton is necessary and urgent.
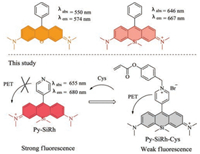
To address above mentioned issues, in this communication, we changed the phenyl group to pyridine expecting to achieve better solubility. Pyridine differs from benzene markedly in the electronic properties and water solubility. We hope that by the introduction of pyridine the hydrophilicity and modifiability of Si-xanthene will be greatly improved. As expected, the innovatively designed pyridine-Si-xanthene (Py-SiRh) shows red-shifted absorption and emission wavelength and much better water solubility. Such properties endow the fluorescent compound with elevated potential in biological applications. Furthermore, Py-SiRh shows excellent intrinsic targeting ability for lysosomes. As we know that lysosomes have played an important role in a series of cellular processes, such as intracellular transportation, metabolism, cell membrane recycling, pathogen defence and apoptosis [11-14]. Lysosome-targeted probes provide a potent tool for the diagnosis of lysosomal associated diseases. More importantly, the nitrogen atom of pyridine offers possibilities for further modification. That is to say Py-SiRh can be exploited for development of NIR fluorescent probe for lysosome-targeted biological imaging applications. To demonstrate the advantage of Py-SiRh, we further synthesized a novel NIR fluorescent probe for the detection of cysteine in cells and living animals (Scheme 1).
|
Download:
|
| Scheme 1. Chemical structures of rhodamine derivatives and Py-SiRh. | |
We started with the synthesis of the easily modifiable NIR platform Py-SiRh. Based on the traditional synthetic method, Py-SiRh could be achieved in 45% yield with the starting materials of 4-pyridinecarboxaldehyde and diaryl silyl ether (Scheme S1 in Supporting information) and were characterized by 1H NMR, 13C NMR and HRMS (Supporting information). Unlike reported Sirhodamines that need DMSO or CH3CN as co-solvent to improve their solubility [15], Py-SiRh exhibited good water solubility in PBS buffer solution (Fig. S1 in Supporting information). In PBS buffer solution, its absorption and emission wavelength were found at 655 nm and 680 nm respectively (5 μmol/L), both of which in the near infrared region. Increasing its concentration, the fluorescence of Py-SiRh would be quenched with emission red-shifted (larger than 30 μmol/L). The absolute quantum yield was measured to be 0.12. Before explore its application in cells and tissues, we tested the biocompatibility of Py-SiRh. An MTS assay was carried out and the result revealed that the viabilities of HeLa cells were not noticeably affected by incubation with different concentration of Py-SiRh (Fig. S2 in Supporting information), suggesting the low cytotoxicity of the present fluorescent platform.
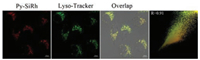
It was reported that Si-rhodamines could selectively localize in lysosomes. Then, we performed the co-staining assays in HeLa cells to test the subcellular localization of Py-SiRh. As shown in Fig. 1, when HeLa cells were co-stained with the Py-SiRh and commercially available Lyso-Tracker green, we observed excellent overlapping images along with high Pearson coefficient (0.91), which indicated that Py-SiRh could specifically localize in lysosomes. The outstanding intrinsic lysosome-targeting ability of Py-SiRh could greatly facilitate the study of lysosome-related pathological and physiological effects.
|
Download:
|
| Fig. 1. Fluorescent images of HeLa cells co-stained with Py-SiRh (2 μmol/L) and Lyso-Tracker green (0.5 μmol/L). Scale bar: 10 μm. | |
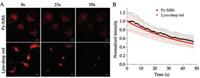
Photostability is another important factor for bioimaging. Hence, we tested the photodegradation of Py-SiRh and commercially available Lyso-Traker deep red in HeLa cells. After irradiated by 5-milliwatt 633 nm laser of the confocal fluorescence microscopy for 50 s, the fluorescence intensity of the Py-SiRh retained ~58% of its initial fluorescence intensity and the commercial dye were photobleached to 53% (Fig. 2). In other words, the photostability of Py-SiRh is comparable to commercial dyes and conventional Si-rhodamine [15]. Moreover, to exhibit deep tissue imaging capability of the present NIR platform, we tested the tissue penetration ability of Py-SiRh using the 80 μm thick frozen kidney tissue slice of rat. The fluorescence signal in different tissue depths were measured through the Z-scan mode of confocal fluorescence microscopy. The results showed that the imaging depths of Py-SiRh were 70 μm (Fig. S3 in Supporting information), indicating the similar ability of the reported Si-rhodamines [15-17].
|
Download:
|
| Fig. 2. (A) Photostability images in HeLa cells stained with Py-SiRh and Lyso-Tracker deep red; (B) Average fluorescence intensity from images of A of different times. Scale bar: 10 μm. | |
As we know, intracellular biothiols including cysteine (Cys), homocysteine (Hcy) and glutathione (GSH) are involved in many physiological and pathological processes like redox homeostasis, cardiovascular and Alzheimer's diseases [18, 19]. So, the detection and discrimination for these biothiols are of great importance, and many probes have been reported [20-27]. With the excellent optical properties, biocompatibility, intrinsic lysosome-targeting ability and imaging ability of Py-SiRh mentioned above, to further demonstrate the easy modifiability of Py-SiRh, we connected the platform with Cys-responsive moiety to build Py-SiRh-Cys for the detection of Cys. The 4-(bromomethyl)phenyl acrylate could be easily linked to the platform through formation of the benzyl pyridinium salt to construct Py-SiRh-Cys (Scheme 1 and Scheme S1 in Supporting information). We hypothesized that the transformation of benzyl pyridinium salt group would quench the fluorescence of Py-SiRh due to the photo-induced electron transfer (PET) effect. After triggered with Cys, acrylate leaved by formation with cyclizing, followed by the methyl phenyl group selfiμmolation to form Py-SiRh with near infrared fluorescence emission turned on.
We initially investigated whether Py-SiRh-Cys can selectively respond to Cys among different biothiols. As shown in Fig. 3, in the absence of Cys, no fluorescence was found in the solution of Py-SiRh-Cys (10 μmol/L) in PBS (pH 7.4, 10 μmol/L) buffer solution; while 80 μmol/L Cys was added at 37 ℃, fluorescence was enhanced obviously (70-fold), and an absorption peak at 650 nm appeared, accompanied by a color change from colorless to light blue, which indicated the transformation from Py-SiRh-Cys to Py-SiRh. When other thiols added to the solution of Py-SiRh-Cys, no obvious fluorescence change was observed, except Hcy caused slight fluorescence enhancement. The kinetics study indicated that fluorescence emission reached maximum intensity within 180 min (Fig. 3B and Fig. S4 in Supporting information). Then, the titration experiment was explored (Fig. 3D and Figs. S5 and S6 in Supporting information). Fluorescence of Py-SiRh-Cys enhanced gradually upon the addition of Cys, and a linearly proportional increment of emission intensity to the concentration of the range of 0–20 μmol/L was disclosed. According to the titration profiles, the detection limit (S/N = 3) of Py-SiRh-Cys toward Cys was calculated to be 32 nmol/L. Meanwhile, the developing of test paper for Cys was also tried. We dipped filter papers into the water solution of Py-SiRh-Cys, after drying up, it showed colorless under daylight and blue emission under 365 nm. Obviously different color towards Cys, Hcy and GSH were found under daylight and 365 nm UV light. These results proved that Py-SiRh-Cys could be served as a dye of test-paper for discrimination of thiol-containing amino acids. Additionally, high resolution mass spectrum was used to prove the response mechanism of Py-SiRh-Cys to Cys (Fig. S7 in Supporting information). A peak at m/z 386.2053 assigned to Py-SiRh was observed; meanwhile, the cyclic addition product of acrylic and Cys (calcd. 174.0230) was found (174.0225) in the mass spectra, which in agreement with the reported mechanism.

|
Download:
|
| Fig. 3. (A) The absorption spectra and fluorescence emission spectra recorded for 10 μmol/L solution of Py-SiRh-Cys upon incubating with Cys (100 μmol/L) for 180 min at 37 ℃ in PBS; (B) Fluorescence emission spectra recorded for 10 μmol/L solution of Py-SiRh-Cys upon incubating with Cys (50 μmol/L) for varying time intervals (0–175 min) at 37 ℃ in PBS; (C) Fluorescence response of Py-SiRh-Cys toward various biologically relevant species (100 μmol/L), GSH (0.5 μmol/L). Inset: Paper imaging of Cys, Hcy and GSH under daylight and 365 nm UV light; (D) Fluorescence spectra changes of Py-SiRh-Cys (10 μmol/L) treated with the increasing concentrations of Cys. | |
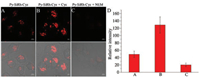
The desirable fluorescence properties of Py-SiRh-Cys for Cys prompted us to study its intracellular application. The imaging experiment of Py-SiRh-Cys for detection of cysteine in living cells was conducted. As shown in Fig. 4, the HeLa cells showed a weak red fluorescence when only treated with the probe, due to the intracellular Cys existence. A stronger red fluorescence was observed after treated with extra cysteine (100 μmol/L). When the HeLa cells were pre-treated with N-ethylmaleimide (NEM) (100 μmol/L), a well-known thiol-blocking reagent, no fluorescence signal is detected. Thus, Py-SiRh-Cys displays an excellent ability for monitoring the cysteine in HeLa cells. Meanwhile, the MTS assay and subcellular localization experiment were carried out and the result revealed that Py-SiRh-Cys exhibited low cytotoxicity as well as good lysosome-targeting ability (Pearson coefficient 0.91).
|
Download:
|
| Fig. 4. (A–C) Fluoresceance images of HeLa cells: (A) Cells were incubated with probe Py-SiRh-Cys (2 μmol/L) for 15 min, scale bar is 10 μm; (B) Cells were incubated with probe Py-SiRh-Cys (10 μmol/L) for 15 min and then treated with cysteine (100 μmol/L) for 30 min, scale bar is 5 μm; (C) Cells were pre-treated with NEM (100 μmol/L) for 30 min and then incubated with probe Py-SiRh-Cys (10 μmol/L) for 15 min, scale bar is 10 μm. (D) Average fluorescence intensity from images of A–C. | |
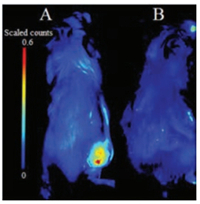
Encouraged by the imaging results in cells, we attempted to examine whether the Py-SiRh-Cys could perform well in B16 tumor-bearing Balb/c mouse. Tumor-bearing Balb/c mice were prepared by subcutaneous injection of B16 cells into the right rear buttock of the Balb/c mice. As shown in Fig. 5, when the Py-SiRhCys was injected into the tumour region, a strong NIR fluorescent signal could be detected, and when the tumor-bearing Balb/c mouse was pretreated with NEM, a well-known inhibitor of cysteine in the tumor region, no obvious fluorescence signal was detected suggesting this NIR fluorescent probe performed well for the detection of Cys in vivo. Especially, it demonstrated the potential application of Py-SiRh as a novel NIR fluorescent probe can be modified flexibly and widely used in medical diagnostic in vivo.
|
Download:
|
| Fig. 5. Fluorescence images in B16 tumor-bearing Balb/c mouse model. (a) Mouse were incubated with probe Py-SiRh-Cys (0.1 μmol/L, 50 μL) in tumor region, and then imaged immediately; (b) Mouse were incubated with NEM (2 μmol/L, 50 mL) in tumor region for 25 min, and incubated with probe Py-SiRh-Cys (0.1 μmol/L, 50 mL), then imaged immediately. | |
In summary, we have developed a novel Pyridine-Si-Xanthene Py-SiRh as a near-infrared fluorescent platform, which shows good solubility and intrinsic targeting ability for lysosomes. Especially, the platform was extended to construct a lysosomes-targeting probe for Cys, which capable of imaging cysteine in cells and in vivo. Py-SiRh showed good modifiability and red-shifted emission wavelength compared to traditional Si-rhodamines. The present study indicated that the Py-SiRh is an excellent fluorescent platform to study lysosomal cell death related the pathological process of cancers and early diagnosis of cancers, more application are in progress.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (Nos. 21572147 and 21877082). We also thank the Comprehensive Training Platform of Specialized Laboratory, College of Chemistry, Sichuan University for sample analysis.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2019.03.017.
| [1] |
(a) R.N. Germain, E.A. Robey, M.D. Cahalan, Science 336 (2012) 1676-1681; (b) R. Weissleder, M.J. Pittet, Nature 452 (2008) 580-589. |
| [2] |
J.B. Grimm, A.K. Muthusamy, Y. Liang, T.A. Brown, L.D. Lavis, Nat. Methods 14 (2017) 987-994. DOI:10.1038/nmeth.4403 |
| [3] |
(a) L.D. Lavis, Annu. Rev. Biochem. 86 (2017) 825-843; (b) H. Zhou, Y. Xiao, X. Hong, Chin. Chem. Lett. 29 (2018) 1425-1428; (c) L. Yuan, W. Lin, Y. Yang, H. Chen, J. Am. Chem. Soc. 134 (2012) 1200-1211; (d) W. Chen, Y. Pan, J. Chen, et al., Chin. Chem. Lett. 29 (2018) 1429-1435; (e) S.A. Hilderbrand, R. Weissleder, Curr. Opin. Chem. Biol. 14 (2010) 71-79; (f) J. Xu, L. Shang, Chin. Chem. Lett. 29 (2018) 1436-1444; (g) M.Y. Wu, K. Li, C.Y. Li, J.T. Hou, X.Q. Yu, Chem. Commun. 50 (2014) 183-185; (h) D. Wu, Y. Shen, J. Chen, et al., Chin. Chem. Lett. 28 (2017) 1979-1982; (i) L. Yuan, W. Lin, K. Zheng, et al., Chem. Soc. Rev. 42 (2013) 622-661; (j) L. Yuan, W. Lin, K. Zheng, et al., Acc. Chem. Res. 46 (2013) 1462-1473. |
| [4] |
(a) Y. Sun, J. Liu, X. Lv, et al., Angew. Chem. Int. Ed. 51 (2012) 7634-7636; (b) F. Deng, Z.C. Xu, Chin. Chem. Lett. (2018), doi: 10.1016/j.cclet.2018.12.012. |
| [5] |
M. Fu, Y. Xiao, X. Qian, D. Zhao, Y. Xu, Chem. Commun. 15 (2008) 1780-17824. |
| [6] |
Y. Koide, Y. Urano, K. Hanaoka, T. Terai, T. Nagano, ACS Chem. Biol. 6 (2011) 600-608. DOI:10.1021/cb1002416 |
| [7] |
T. Egawa, K. Hanaoka, Y. Koide, et al., J. Am. Chem. Soc. 133 (2011) 14157-14159. DOI:10.1021/ja205809h |
| [8] |
T.E. McCann, N. Kosaka, Y. Koide, et al., Bioconjugate Chem. 22 (2011) 2531-2538. DOI:10.1021/bc2003617 |
| [9] |
Y. Koide, Y. Urano, K. Hanaoka, et al., J. Am. Chem. Soc. 134 (2012) 5029-5031. DOI:10.1021/ja210375e |
| [10] |
Y. Koide, Y. Urano, K. Hanaoka, T. Terai, T. Nagano, J. Am. Chem. Soc. 133 (2011) 5680-5682. DOI:10.1021/ja111470n |
| [11] |
(a) S. Goldstein, J. Lind, G. Merényi, Chem. Rev. 105 (2005) 2457-2470; (b) F.Q. Schafer, G.R. Buettner, Free Radic. Biol. Med. 30 (2001) 1191-1212. |
| [12] |
R. Radi, J. Biol. Chem. 288 (2013) 26464-26472. DOI:10.1074/jbc.R113.472936 |
| [13] |
(a) J.P. Luzio, P.R. Pryor, N.A. Bright, Nat. Rev. Mol. Cell Biol. 8 (2007) 622-632; (b) P. Ning, W. Wang, M. Chen, Y. Feng, X. Meng, Chin. Chem. Lett. 28 (2017) 1943-1951. |
| [14] |
(a) P. Saftig, J. Klumperman, Nat. Rev. Mol. Cell Biol. 10 (2009) 623-635; (b) T. Kallunki, O.D. Olsen, M. Jaattela, Oncogene 32 (2013) 1995-2004. |
| [15] |
(a) Y. Huo, J. Miao, L. Han, et al., Chem. Sci. 8 (2017) 6857-6864; (b) K. Umezawa, M. Kamiya, Y. Urano, Angew. Chem. Int. Ed. 57 (2018) 9346-9350. |
| [16] |
H. Zhang, J. Liu, L. Wang, et al., Biomaterials 158 (2018) 10-22. DOI:10.1016/j.biomaterials.2017.12.013 |
| [17] |
H. Zhang, J. Liu, C. Liu, et al., Biomaterials 133 (2017) 60-69. DOI:10.1016/j.biomaterials.2017.04.023 |
| [18] |
(a) M. Kim, S.K. Ko, H. Kim, I. Shin, J. Tae, Chem. Commun. 49 (2013) 7959-7961; (b) T. Peng, X. Chen, L. Gao, et al., Chem. Sci. 7 (2016) 5407-5413. |
| [19] |
(a) J. Hu, N. Wong, M. Lu, et al., Chem. Sci. 7 (2016) 2094-2099; (b) M.Y. Wu, T. He, K. Li, et al., Analyst 138 (2013) 3018-3025; (c) Y. Liu, K. Li, M.Y. Wu, et al., Chem. Commun. 51 (2015) 10236-10239. |
| [20] |
(a) W. Lin, L. Long, L. Yuan, et al., Org. Lett. 10 (2008) 5577-5580; (b) J. Liu, Y. Sun, H. Zhang, et al., Chem. Sci. 5 (2014) 3183-3188; (c) L. Song, L.M. Ma, Q. Sun, et al., Chin. Chem. Lett. 27 (2016) 330-334; (d) Y. Liu, K. Li, K.X. Xie, Chem. Commun. 52 (2016) 3430-3433. |
| [21] |
(a) K.S. Lee, T.K. Kim, J.H. Lee, H.J. Kim, J.I. Hong, Chem. Commun. 46 (2008) 6173-6175; (b) P. Zhang, Z. Guo, C. Yan, W. Zhu, Chin. Chem. Lett. 28 (2017) 1952-1956. |
| [22] |
(a) Y. Yue, F. Huo, P. Ning, et al., J. Am. Chem. Soc. 139 (2017) 3181-3185; (b) Y. Yang, H. Wang, Y. Wei, et al., Chin. Chem. Lett. 28 (2017) 2023-2026; (c) J. Yang, K. Li, J.T. Hou, et al., Sci. China Chem. 60 (2017) 793-798. |
| [23] |
(a) J.T. Hou, J. Yang, K. Li, K.K. Yu, X.Q. Yu, Sens. Actuators B: Chem. 214 (2015) 92-100; (b) M. Li, P. Cui, K. Li, et al., Chin. Chem. Lett. 29 (2018) 992-994; (c) J.Y. Xie, C.Y. Li, Y.F. Li, et al., Anal. Chem. 88 (2016) 9746-9752. |
| [24] |
(a) J. Liu, Y.Q. Sun, Y. Huo, et al., J. Am. Chem. Soc. 136 (2014) 574-577; (b) Y. Liu, X. Lv, M. Hou, Y. Shi, W. Guo, Anal. Chem. 87 (2015) 11475-11483; (c) Y. He, Z. Li, Q. Jia, et al., Chin. Chem. Lett. 28 (2017) 1969-1974. |
| [25] |
(a) Y. Yue, F. Huo, P. Ning, et al., J. Am. Chem. Soc. 139 (2017) 3181-3185; (b) J. Yang, K. Li, J.T. Hou, et al., ACS Sen. 1 (2016) 166-172. |
| [26] |
(a) K. Dou, Q. Fu, G. Chen, et al., Biomaterials 133 (2017) 82-93; (b) M.Y. Wu, K. Li, J.T. Hou, Z. Huang, X.Q. Yu, Org. Biomol. Chem. 10 (2012) 8342-8347. |
| [27] |
(a) Y. Chen, T. Zhang, X. Gao, et al., Chin. Chem. Lett. 28 (2017) 1983-1986; (b) Z. Lei, Z. Zeng, X. Qian, Y. Yang, Chin. Chem. Lett. 28 (2017) 2001-2004. |
 2019, Vol. 30
2019, Vol. 30 

