b Department of Chemistry, Graduate School of Science, Hiroshima University, Higashi-Hiroshima 739-8526, Japan;
c Center of Physical and Chemistry Test, Shenyang University of Chemical Technology, Shenyang 110142, China;
d College of Pharmaceutical and Biological Engineering, Shenyang University of Chemical Technology, Shenyang 110142, China
Photodynamic therapy (PDT) was usually advocated as anon- invasive medical technique for a damage of tumor tissues [1-3]. Because photosensitizers (PS) transfer energy from light to the molecular oxygen to produce the singlet oxygen (1O2), which can cause oxidative damage of lipids, proteins and DNA in tumor tissues during PDT process [4-7]. Therefore, the singlet oxygen is an important cytotoxic agent in the PDT therapeutic process, and the investigation of generation of the singlet oxygen has attracted increasing interest [8-11]. Generally speaking, the singlet oxygen generation from a PS is controlled by the spin-forbidden electronic transition from a singlet to a triplet state upon irradiation [12]. Introduction of the heavy atom to the fluorescent molecules was found to efficiently increase intersystem crossing (ISC) to generate the singlet oxygen [13-15].
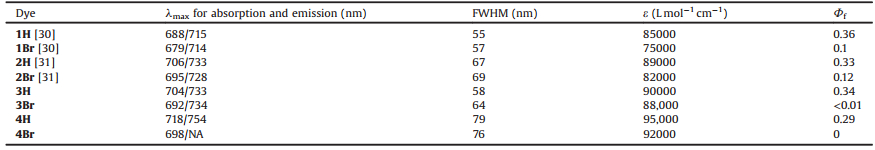
Borondipyrromethenes (BODIPYs) are the classical fluorescent dyes with intense absorption, high quantum yields, and tunable emissionwavelength [16-21]. Such dyes and theirderivatives were widespreadly applied for biological imaging, fluorescent probes, dye-sensitized solar cells and supramolecular chemistry and so forth [22-28]. Take advantage of the attachment of the heavy atoms to the BODIPY core, the modifications could provide the outstanding efficiency of the singlet oxygen generation by Corresponding author. increasing the S1→T1 transition [29]. The pioneer work reported by O'Shea indicated that aza-BODIPY 1Br (labs =679 nm) is an excellent PS (Fig. 1a) [30], but the reserved topic is to be develop NIR-absorbing (> 701 nm) aza-BODIPY-based PS. Recently, our group reported aza-BODIPYs 2H and 2Br with the naphthyl group at 3, 5-positions (Fig. 1a) [31]. In this work, using of the heavyatom effect, we reported synthesis and photophysical properties including the singlet oxygen generation of NIR-absorbing dyes 3Br and 4Br (λabs = 705 nm) with the naphthyl group at 1, 7-positions in aza-BODIPY system for the first time (Fig. 1b).
|
Download:
|
| Fig. 1. The design strategies for the naphthyl-containing aza-BODIPYs at 1, 7- positions. | |
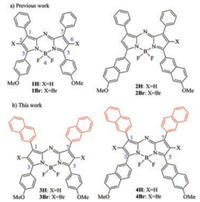
Synthesis of NIR-absorbing dye aza-BODIPY 4Br with four naphthyl groups at 1, 3, 5, 7-positions was outlined in Scheme 1. Utilizing 2-vinylnaphthalene as the starting material, the snuffcol- ored oil 3-(naphthalen-2-yl)-2H-azirine 5 was obtained in 53% yield in the mild condition, comparing to the relatively rigorous condition for the synthetic of analogue (Scheme 1) [32]. For the preparation of the naphthyl-containing pyrrole, 1-(6-methoxy- naphthalen-2-yl)ethanone was employed to react with 5 to providess 2-(6-methoxynaphthalen-2-yl)-4-(naphthalen-2-yl)- 1H-pyrrole 6 in 37% yield in the presence of NaH (Scheme 1) [33]. The dinaphthyl-containing pyrrole 6 showed a typical hydrogen signal δ 8.72 (br s, 1H, N-H) in the 1H NMR spectrum (Supporting information), which is in agreement with that of the reported pyrrole (δ 8.56 (br s, 1H, N-H) [31, 34-37]. aza-BODIPY4H was successfully synthesized from pyrrole 6 in 36% combined yield under HOAc, Ac2O and NaNO2, followed by complexation with BF3·Et2O-Et3N (Scheme 1). Moreover, aza-BODIPY 4H reacted with N-bromosuccinimide to provide the dibromo substituted aza- BODIPY 4Br. To our knowledge, this aza-BODIPY structure is the first to bear a naphthyl group at 1, 7-positions in aza-BODIPY system.

|
Download:
|
| Scheme 1. Synthesis of aza-BODIPY 4Br. | |
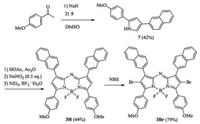
To severelycompare with the photophysical properties, as shown in Scheme 2, aza-BODIPYs 3H and 3Br were synthesized too.
|
Download:
|
| Scheme 2. Synthesis of aza-BODIPY 3Br. | |
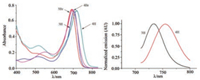
The spectra of absorption and fluorescence of 3 and 4 are shown and outlined in Fig. 2 and Table 1, and the excitation wavelength (λex) is 700 nm. Due to the heavy atom effect, both compounds 3Br (Φf < 0.01) and 4Br (Φf=0) are very weakly fluorescent, in comparison with 3H (Φf= 0.34) and 4H (Φf = 0.29). Dye 3H absorbs and emits at 704 and 733 nm, respectively. Changing the phenyl to naphthyl group at 3, 5-positions in 3H, aza-BODIPY 4H shows long- wavelength absorption and emission waves (λabs/λem = 718/754 nm). The absorption maximum of 4Br is blue-shifted to 698 nm comparing to that of the corresponding parent aza-BODIPY 4H (λabs =718 nm). NIR-absorbing Dyes 3 and 4 have high extinction coefficients (ε = 88000-95000 Lmol-1 cm-1). Attaching of the naphthyl group at 3, 5-positions provides a broader full width at half maximum for 4Br (FWHM = 76 nm) compared with 3Br (FWHM = 64 nm).
|
Download:
|
| Fig. 2. Absorption and fluorescence spectra of 3 and 4 in CH2Cl2 at 298 K. λex = 700 nm for 3 and 4. | |
|
|
Table 1 Photophysical properties of aza-BODIPYs 3 and 4 in CH2Cl2 at 298 K. |
In comparison with the maximum absorption of aza-BODIPY 2H bearing the phenyl groups at 1, 3-positions (λabs =701 nm) [31], a substituent change from phenyl to naphthyl group in dye 4H (λabs = 718 nm) leads to be 12 nm of bathochromic shift. However, only attaching naphthyl groups at 1, 7-positions or 3, 5-positions in 1H, their photophysical properties 2H and 3H are nearly same, except the FWHM. Additionally, the molecular geometries of aza- BODIPYs 1H, 2H and 4H were optimized by using density functional theory (DFT) at the B3LYP/6-31 G(d) level of theory [38]. The calculated HOMO and LUMO orbital energy levels are summarized in Fig. 3. The HOMO and LUMO were mainly localized in the aza-BODIPY core. Changing the substituent from phenyl to naphthyl group resulted in a remarkable bathochromic shift (λabs = 681 nm for 1H, λabs = 701 nm for 2H, λabs = 711 nm for 4H), owing to a narrowing of the HOMO-LUMO band gap for the lowest-energy absorption bands in dye 4H (1.95 eV) relative to those of dye 1H (2.06 eV) or dye 2H (1.97 eV), according to MO calculations (Fig. 3).

|
Download:
|
| Fig. 3. Frontier molecular orbitals of aza-BODIPYs 1, 2 and 4 at the B3LYP/6-31 G(d) level with Gaussian 09. For 1, 2 and 4, HOMO/LUMO (eV) = -5.33/-3.27, -4.99/-3.02, and -4.99/-3.04, respectively. | |
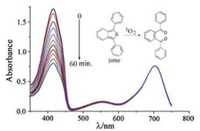
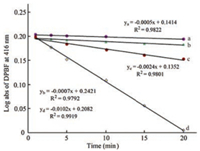
The investigation of the singlet oxygen generation was performed to assess the ability of naphthyl-containing aza- BODIPYs 3Br and 4Br as a PS in toluene. To simulate a deep tissue penetration and to reduce the normal cell-damage, the monochromatic light [39-41] at 690 nm by using a 150W xenon lamp at 0.5 mW/cm2 was selected to irradiate the toluene solution. Singlet oxygen generation was estimated by 1, 3-diphenylisobenzofuran (DPBF), a singlet oxygen indicator [42]. The experiments were performed at initial concentrations of 6.5 × 10-6 mol/L of 3 and 4, and 6 × 10-5 mol/L of DPBF. When the oxidation of DPBF with the reactive singlet oxygen happened, the absorption maximum of DPBF at 411 nm was found to be decreased. The results demonstrate that the singlet oxygen generation of 4Br with the dibromo groups at 2, 6-positions (Figs. 4 and 5) was more effective than that of 4H. A 14.5-fold rate enhancement is observed for 4Br compared to 4H (Fig. 5 and Fig. S1 in Supporting information). And, the singlet oxygen generation of 4Br (4.2-fold rate) is more effective than that of 3Br (Fig. 5 and Fig. S1). Moreover, one notices that aza-BODIPY 4Br with the naphthyl groups at 1, 7-positions is a stronger PS than 2Br with the phenyl groups at 1, 7-positions, having a 2.2-fold rate enhancement for generating the singlet oxygen (Fig. S2 in Supporting information) [31]. Additionally, no photobleaching of 4Br was found during this experiment, based on the absorption intensity (λabs = 705 nm in toluene) (Fig. 4). These results indicated that the NIR PS 4Br was able to be used for the generation of the singlet oxygen.
|
Download:
|
| Fig. 4. DPBF (initial concentration at 6 × 10-5 mol/L) degradation profile in toluene by aza-BODIPY 4Br (6.5×10-6 mol/L) under the light. Monochromatic light (690 nm at 0.5 mW/cm2) was used. The figure displays time-dependent decrease (0, 1, 3, 5, 10, 15, 20, 25, 30, 40, 50 and 60 min) of the absorbance at 416 nm by oxidation of DPBF with 4Br. | |
|
Download:
|
| Fig. 5. Time-dependent decrease (0, 1, 3, 5, 10, 15 and 20 min) of log absorbance at 416 nm by oxidation of DPBF with aza-BODIPY 3H (line a), 4H (line b), 3Br (line c) and 4Br (line d). | |
In summary, utilizing 2-vinylnaphthalene as the starting material, aza-BODIPYs bearing the naphthyl groups at 1, 7- positions were prepared. To attaching naphthyl group at 1, 7- positions, aza-BODIPYs show long-wavelength absorption (λabs > 692 nm) and emission (λem > 733 nm) in the near-infrared region. NIR naphthyl-containing aza-BODIPYs possess high extinction coefficients. The singlet oxygen generation of the dibromo substituted aza-BODIPY as a PS was more effective and had about 14.5-fold rate enhancement compared to the parent aza-BODIPY. No photobleaching of naphthyl-containing aza-BODIPY was observed and such NIR aza-BODIPY could be used for the singlet oxygen generation.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21542004, 21372156), Young and middle-aged scientific and technological innovation talents of Shenyang Science and Technology Bureau (No. RC170140), Liaoning Province Natural Science Foundation (No. 20170540721), Basic research on the application of Industrial Development of Shenyang Science and Technology Bureau (No. 18013027), and the Distinguished Professor Project of Liaoning province. We thank the Chinese Scholarship Council (No. 20183058) and State Administration ofForeign Experts Affairs (No. P183008050) Programme for financial support. We also thank Prof. Yohsuke Yamamoto (Hiroshima University) for his help.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2019.02.016.
| [1] |
A.G. Leach, K.N. Houk, Chem. Commun. (2002) 1243-1255. |
| [2] |
M. Stratakis, M. Orfanopoulos, Tetrahedron 56 (2000) 1595-1616. DOI:10.1016/S0040-4020(99)00950-3 |
| [3] |
S.B. Brown, E.A. Brown, I. Walker, Lancet. Oncol. 5 (2004) 497-508. DOI:10.1016/S1470-2045(04)01529-3 |
| [4] |
D. Dolmans, D. Fukumura, R.K. Jain, Nature Rev. Cancer 3 (2003) 380-387. DOI:10.1038/nrc1071 |
| [5] |
R. Bonnett, Chem. Soc. Rev. 24 (1995) 19-33. DOI:10.1039/cs9952400019 |
| [6] |
M. Ethirajan, Y. Chen, P. Joshi, et al., Chem. Soc. Rev. 40 (2011) 340-362. DOI:10.1039/B915149B |
| [7] |
T.C. Jorgenson, W. Zhong, T.D. Oberley, Cancer Res. 73 (2013) 6118-6123. DOI:10.1158/0008-5472.CAN-13-1117 |
| [8] |
J.V. Frangioni, Curr. Opin. Chem. Biol. 7 (2003) 626-634. DOI:10.1016/j.cbpa.2003.08.007 |
| [9] |
H. Li, P. Wang, W.H. Zhu, et al., Biomaterials 139 (2017) 30-38. DOI:10.1016/j.biomaterials.2017.05.030 |
| [10] |
X. Li, S. Lee, J. Yoon, Chem. Soc. Rev. 47 (2018) 1174-1188. DOI:10.1039/C7CS00594F |
| [11] |
S. Erbas-Cakmak, E.U. Akkaya, Angew. Chem. Int. Ed. 52 (2013) 11364-11368. DOI:10.1002/anie.v52.43 |
| [12] |
J. Michl, J. Am. Chem. Soc. 118 (1996) 3568-3579. DOI:10.1021/ja9538391 |
| [13] |
N. Adarsh, R.R. Avirah, D. Ramaiah, Org. Lett. 12 (2010) 5720-5723. DOI:10.1021/ol102562k |
| [14] |
T. Yogo, Y. Urano, Y. Ishitsuka, et al., J. Am. Chem. Soc. 127 (2005) 12162-12163. DOI:10.1021/ja0528533 |
| [15] |
G. Ulrich, R. Ziessel, A. Harriman, Angew. Chem. Int. Ed. 47 (2008) 1184-1201. DOI:10.1002/(ISSN)1521-3773 |
| [16] |
A. Loudet, K. Burgess, Chem. Rev. 107 (2007) 4891-4932. DOI:10.1021/cr078381n |
| [17] |
H. Lu, J. Mack, Z. Shen, et al., Chem. Soc. Rev. 43 (2014) 4778-4823. DOI:10.1039/C4CS00030G |
| [18] |
S.O. McDonnell, M.J. Hall, D.F. O'Shea, et al., J. Am. Chem. Soc. 127 (2005) 16360-16361. DOI:10.1021/ja0553497 |
| [19] |
M.J. Hall, L.T. Allen, D.F. O'Shea, Org. Biomol. Chem. 4 (2006) 776-780. DOI:10.1039/b514788c |
| [20] |
J. Feng, J.Y. Shao, H. Nie, Z. Gong, Y.W. Zhong, Chin. Chem. Lett. 29 (2018) 385-389. DOI:10.1016/j.cclet.2017.11.014 |
| [21] |
N. Adarsh, M.S. Krishnan, D. Ramaiah, Anal. Chem. 86 (2014) 9335-9342. DOI:10.1021/ac502849d |
| [22] |
K. Umezawa, Y. Nakamura, H. Makino, et al., J. Am. Chem. Soc. 130 (2008) 1550-1551. DOI:10.1021/ja077756j |
| [23] |
S.G. Awuah, J. Polreis, V. Biradar, et al., Org Lett. 13 (2011) 3884-3887. DOI:10.1021/ol2014076 |
| [24] |
Y.W. Wang, A.B. Descalzo, Z. Shen, et al., Chem.-Eur. J. 16 (2010) 2887-2903. DOI:10.1002/chem.200902527 |
| [25] |
T. Li, X. Ma, Dye Pigm. 148 (2018) 306-312. DOI:10.1016/j.dyepig.2017.09.036 |
| [26] |
L. Zhou, Z. Jin, X. Fan, et al., Chin. Chem. Lett. 29 (2018) 1500-1502. DOI:10.1016/j.cclet.2018.07.018 |
| [27] |
D. Li, F. Lu, H. Tian, et al., J. Am. Chem. Soc. 140 (2018) 1916-1923. DOI:10.1021/jacs.7b12800 |
| [28] |
Y. Xia, X. Liu, D. Wang, et al., Chin. Chem. Lett. 29 (2018) 1517-1520. DOI:10.1016/j.cclet.2018.01.054 |
| [29] |
P. Majumdar, X. Yuan, S. Li, et al., J. Mater. Chem. B 2 (2014) 2838-2854. DOI:10.1039/C4TB00284A |
| [30] |
A. Gorman, J. Killoran, D.F. O'Shea, et al., J. Am. Chem. Soc. 126 (2004) 10619-10631. DOI:10.1021/ja047649e |
| [31] |
X.D. Jiang, D. Xi, B. Le Guennic, et al., Tetrahedron 71 (2015) 7676-7680. DOI:10.1016/j.tet.2015.07.068 |
| [32] |
Timén T.Å., E. Risberg, P. Somfai, Tetrahedron Lett. 44 (2003) 5339-5341. DOI:10.1016/S0040-4039(03)01205-X |
| [33] |
W. Zhao, E.M. Carreira, Chem. -Eur. J. 12 (2006) 7254-7263. DOI:10.1002/(ISSN)1521-3765 |
| [34] |
X.D. Jiang, J. Zhao, D. Xi, et al., Chem. -Eur. J. 21 (2015) 6079-6082. DOI:10.1002/chem.v21.16 |
| [35] |
X.D. Jiang, X. Liu, T. Fang, et al., Dyes Pigm. 146 (2017) 438-444. DOI:10.1016/j.dyepig.2017.07.038 |
| [36] |
X.D. Jiang, J. Guan, J. Zhao, et al., Dyes Pigm. 136 (2017) 619-626. DOI:10.1016/j.dyepig.2016.09.019 |
| [37] |
X.D. Jiang, S. Li, B. Le Guennic, et al., Phys. Chem. Chem. Phys. 18 (2016) 32686-32690. DOI:10.1039/C6CP05705E |
| [38] |
M.J. Frisch, G.W. Trucks, H.B. Schlegel, et al., Gaussian 09W, Revision A.1, Gaussian Inc., Wallingford, CT (2009). |
| [39] |
L. Huang, X. Cui, J. Zhao, et al., Chem.-Eur. J. 19 (2013) 17472-17482. DOI:10.1002/chem.201302492 |
| [40] |
K. Xu, J. Zhao, X. Cui, et al., J. Phys. Chem. A 119 (2015) 468-481. DOI:10.1021/jp5111828 |
| [41] |
Z. Wang, J. Zhao, A. Barbon, et al., J. Am. Chem. Soc. 139 (2017) 7831-7842. DOI:10.1021/jacs.7b02063 |
| [42] |
K. Gollnick, A. Griesbeck, Tetrahedron 41 (1985) 2057-2068. DOI:10.1016/S0040-4020(01)96576-7 |
 2019, Vol. 30
2019, Vol. 30