b State Key Laboratory of Marine Environmental Science, Xiamen University, Xiamen 361005, China;
c Department of Anesthesiology, Xiamen Maternal and Children Hospital, Xiamen 361003, China;
d Key Laboratory of Sensor Analysis of Tumor Marker, Ministry of Education, College of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, China
Direct exposure to ultraviolet (UV) light is closely related to various harmful effects [1-3], ranging from skin injures to cancer originated from DNA damage. Recent years, some studies reported that blue light are also detrimental to humans [4, 5], for example, the bluelight could cause photochemical lesions tohuman retinalwithin the intensity range of the natural light [6]. Furthermore, blue light is responsible for the solar retinitis and may play a role in age-related macular degeneration. Importantly, the harmful effects ofblue lights generated from the electronic display devices should also be careful [7]. Thus, the development of new UV and blue light shielding materials has been received much attention [8].
In the past few years, there has been an increased awareness of the importance to develop UV shielding materials. A variety of materials have been used to prevent UV lesions. Organic molecules like avobenzone or oxybenzone have been used as a UV absorber for many years, but the self-degradation limits their usage time. Inorganic materials such as zinc oxide (ZnO) and titanium oxide (TiO2) have been used intensively for UV shielding [9-12]. However, photocatalytic properties and self-degradations of the ZnO and TiO2 based absorbers also hindered their applications [13, 14]. Other materials, e.g., graphene oxide-poly(vinyl alcohol) composite film and lanthanide complex functionalized cellulose nanopaper were also reported for UV shielding [15, 16]. Whereas, the excellent UV-filtering capability of these films was obtained by sacrificing the visible light transmittance. Therefore, fabricating UV and blue light blocking materials with good photostability and high transparency to the rest of visible light still remains a challenge and is urgently needed to be developed. Recently, because of the outstanding performance in photovoltaic applications, lead halide perovskite APbX3 (where A = CH3NH3+, (NH2)2CH+ and Cs+, X = Cl-, Br- and I-) has become the most noticeable materials [17-22]. These perovskite nanocrystals exhibit intriguing features [23], such as easy tunable band gap, sharp optical absorption edges and high quantum efficiency with narrow emission spectra. These nanocrystals have been studied extensively for various optical applications, especially light emitting diodes and lasers [24-27]. Post modification of perovskite nanocrystals by anion exchange enables the absorbance band gap tuned from ultraviolet to near infrared spectra [28, 29]. In addition, the perovskite nanocrystals show large absorption range, which offers the great potential for UV and blue light shielding applications. Although the tunable absorption-band edge of perovskite nanocrystals has already been realized, there have not been reports on developing UV and blue light blocking material with tunable absorption-band edge.
Herein, we aim to the development of a simple and easy way to fabricate UV and blue light blocking material by mixing pervoskite nanocrystals and ethyl cellulose (EC). In this study, EC was used as a host material for the CsPb(Cl/Br)3 pervoskite nanocrystals. By tuning the ratio of Br to Cl, the blocked wavelength range could be easily controlled. Using the sharp absorption edges, the material possesses excellent light blocking ability in the range of 200– 460 nm and maintains high transparency (95%) to visible light in the range beyond blue light.
Here, CsPb(Cl/Br)3 nanocrystals were synthesized by the hot injection method as the literature [22] with some adaptions, and the samples with different Cl/Br were obtained by anion exchange [28, 29]. Typically, 5 mL octadecene, 0.188 mmol PbX2 (X = Cl, Br), 0.5 mL oleylamine and 0.5 mL oleic acid were loaded into a 25 mL four neck flask, degassed at 120 ℃ for 30 min and heated to 150 ℃ under nitrogen flow, 0.4 mL cesium sterate solution was quickly injected. After 5 s, the reaction mixture was cooled by the ice-water bath. The resultant nanocrystals were precipitated via centrifugation (10, 000 rpm, 20 min). And the separated nanocrystals were redispersed in 10 mL toluene for further use. The concentration of the obtained nanocrystal solution was then denoted as 100%. Nanocrystal solutions with different concentrations were obtained by adjusting the volume of toluene. Using the anion exchange reaction between different halide perovskite nanocrystals, perovskite nanocrystals with different Cl/Br ratio could be easily synthesized. CsPbCl2.75Br0.25, CsPbCl2.5Br0.5 and CsPbCl2Br were obtained by mixing different volume of CsPbCl3 and CsPbBr3 nanocrystal in the ratio of 11:1, 5:1 and 2:1, respectively. For the fabrication of EC-CsPb(Cl/Br)3 films, 0.2 g EC were added into 5 mL perovskite nanocrystals colloid, and the mixture were ultrasonic for 60 min to form a homogenous colloid. The colloid was spin coated onto an optical glass, and the toluene was evaporated in room temperature.
High resolution transmission electron microscopy (HRTEM) images were obtained by using Tecnai F30 (Philips-FEI, Holland). The absorption and transmittance spectra were recorded using UV 2450 (Shimadzu, Japan). The fluorescence spectra were recorded using F-4500 (Hitachi, Japan) under excitation wavelength of 385 nm, and the excitation and emission slits were set to be 2.5 and 5 nm. The photostability of the sample was evaluated by collecting the transmittance spectra after the irradiation with a UV lamp (365 nm, 100 W/m2) for 0, 2, and 6 days.
Fig. 1 shows the HRTEM images of the nanocrystals with different Cl/Br ratio. The nanocrystals are symmetric cubic, uniform dispersed and the average size is approximately 10 nm. Obviously, the anion exchange reaction between CsPbCl3 and CsPbBr3 preserves the pristine crystal structures.

|
Download:
|
| Fig. 1. TEM images of the CsPb(Cl/Br)3 nanocrystals with different Br/Cl ratio. (a) CsPbCl3, (b) CsPbCl2.75Br0.25, (c) CsPbCl2.5Br0.5, (d) CsPbCl2Br and (e) CsPbBr3. The CsPb(Cl/Br)3 is prepared from anion exchange reaction. | |
The UV–vis spectra of samples of different Br/Cl ratio are illustrated in Fig. 2a. The absorption edges of the perovskite nanocrystals show a red shift with the increase of Br/Cl anion, demonstrating the adsorption edges can be well adjusted by the change of the Br/Cl ratio. The color of their colloid turns slight yellow with the increase proportion of Br (Inset in Fig. 2a, from left to right), which also indicates the red shift of absorbance edge.

|
Download:
|
| Fig. 2. (a) The UV–vis absorbance spectra of CsPb(Cl/Br)3 dispersed in toluene (the concentration of the CsPb(Cl/Br)3 is 25%). Inset is the digital picture of the prepared CsPb(Cl/Br)3 with different Cl/Br ratio. (b) UV–vis transmittance spectrum of EC film. Inset is the digital photo of the EC film. | |
Here, the EC is selected as the host for the following reasons: (1) EC is dissolved in toluene, in which perovskite nanocrystals could be well dispersed; (2) The hydrophobic property of EC improves the moisture resistance of the light blocking film; (3) EC is a well commercialized product that could be easily obtained; (4) The EC is free of absorption in the range from 280 nm to 800 nm, which enable the film retains high transparency in the visible light range (Fig. 2b); (5) The perovskite NCs retain their optical properties during the fabricating process, as shown by their photoluminescence spectra (Figs. S1 and S2 in Supporting information).
The light shielding properties of the EC-nanocrystals films were characterized from their UV–vis transmittances. The UV–vis transmittance spectra of EC-CsPbCl2.5Br0.5 film for different concentrations are evidenced in Fig. 3a. Results indicate that with the increased concentration of nanocrystals, the UV and blue lights in the range of 280–460 nm are gradually blocked, while the transparency keeps constant in 480–800 nm. When the concentration of CsPbCl2.5Br0.5 is up to 100%, the UV and blue lights in the range of 280–460 nm can be completely blocked, indicating the excellent UV and blue light shielding performance of CsPbCl2.5Br0.5.To testify the tunable light blocking prosperity, the UV–vis transmittance spectra of EC-CsPb(Cl/Br)3 films were investigated. As shown in Fig. 3b, the light blocking range shifts from 400 nm to 500 nm by changing the Cl/Br ratio. It should be noted that in Fig. 3b, the concentration of each CsPb(Cl/Br)3 is 190%, 160%, 130%, 100% and 70%, respectively, since the absorbance coefficient is increased with the increasing Br proportion. The adjusting of Cl/Br ratio did not affect the transparency of the EC-nanocrystals film as shown in the digital photographs of EC-CsPb(Cl/Br)3 films in Fig. 3b.

|
Download:
|
| Fig. 3. (a) Concentration-dependent UV–vis transmittance spectra of ECCsPbCl2.5Br0.5 films. Inset graph is the digital photo of the EC-CsPbCl2.5Br0.5 films with concentration of 5%, 25%, 50%, 75%, and 100% (from left to right). (b) Br/Cl ratiodependent UV–vis transmittance spectra of the EC-CsPb(Cl/Br)3 films. Inset is the digital photo of the EC-CsPb(Cl/Br)3 films with different Br/Cl ratio. | |
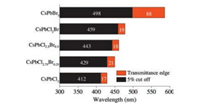
The wavelength for the 5% and 90% cutoff transmittance were selected to evaluate the tunable light blocking properties of ECnanocrystals film (Fig. 4). The EC-CsPbCl3 and EC-CsPbCl2.75Br0.25 films show transmittance of 5% at 412 nm and 429 nm, with a transmittance edge of 17 and 21 nm, respectively. The ECCsPbCl2Br film exhibits a transmittance of 5% at 459 nm and 90% at 478 nm (the wavelength for the 5% cutoff transmittance 459 nm plus the transmittance edge 19 nm), which makes it excellent for UV and blue light shielding. The adjusting of Cl/Br ratio of the nanocrystals allows for the control of light blocking range, making the EC-CsPb(Cl/Br)3 films suitable for various optical applications, such as light blocking and optical filters. The EC-CsPbBr3 shows transmittance of 5% at 498 nm, however, with a broad transmittance edge of 88 nm, indicating the reduced performance for the finely control of blocking range.
|
Download:
|
| Fig. 4. The wavelength for 5% cutoff transmittance and the transmittance edge of the EC film containing different Br/Cl ratio of CsPb(Cl/Br)3 nanocrystals. | |
Photostability is one of the most important issues in real application of light blocking materials, and the degradation of the lead halide perovskite is considered as a critical issue in various applications [30-34]. In this point of view, the stability of the film is highly demanded to be evaluated. The UV–vis transmittance spectra before and after under 6 days UV exposure under ambient environment is shown in Fig. S2 (Supporting information), and it is clearly show that the stability of the film is quite well, which might be benefit from to the excellent photostability of the CsPbCl2.5Br0.5 [23] and the protection of EC matrix.
In summary, we report a CsPb(Cl/Br)3 nanocrystals based UV and blue light blocking material. The perovskite nanocrystals retained their photochemical and physical properties during the fabricating process, and the light blocking range is tunable by changing the Cl/Br ratio. The materials show high transparency beyond the blue light range (>95%), which offer the great advantage in various applications.
AcknowledgmentsThis research was financially supported by the National Nature Scientific Foundation of China (No. 21675133) and OESACLS201902, which are gratefully acknowledged.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.12.028.
| [1] |
J. Wu, S. Seregard, P.V. Algvere, Surv. Ophthalmol. 51 (2006) 461-481. DOI:10.1016/j.survophthal.2006.06.009 |
| [2] |
R. Sarkany, Medicine 45 (2017) 444-447. DOI:10.1016/j.mpmed.2017.04.009 |
| [3] |
A.R. Young, J. Claveau, A.B. Rossi, J. Am. Acad. Dermatol. 76 (2017) S100-S109. DOI:10.1016/j.jaad.2016.09.038 |
| [4] |
P.V. Algvere, J. Marshall, S. Seregard, Acta Ophthalmol. Scand. 84 (2006) 4-15. |
| [5] |
Z. Yu, S. Qiu, X. Chen, et al., Neuroscience 270 (2014) 158-167. DOI:10.1016/j.neuroscience.2014.04.013 |
| [6] |
T. Ueda, T. Nakanishi-Ueda, H. Yasuhara, R. Koide, W.W. Dawson, Exp. Eye Res. 89 (2009) 863-868. DOI:10.1016/j.exer.2009.07.018 |
| [7] |
I. Jaadane, P. Boulenguez, S. Chahory, et al., Free Radical Bio. Med. 84 (2015) 373-384. DOI:10.1016/j.freeradbiomed.2015.03.034 |
| [8] |
C.B. Dipl-Biol, M. Schulz, T. Laube, J. Cataract Refr. Surg. 34 (2008) 1161-1166. DOI:10.1016/j.jcrs.2008.03.039 |
| [9] |
C. Han, F. Wang, C. Gao, et al., J. Mater. Chem. C Mater. Opt. Electron. Devices 3 (2015) 5065-5072. DOI:10.1039/C4TC02880E |
| [10] |
L. Wallenhorst, L. Gurău, A. Gellerich, et al., Appl. Surf. Sci. 434 (2018) 1183-1192. DOI:10.1016/j.apsusc.2017.10.214 |
| [11] |
X. Wu, J. Wang, G. Zhang, et al., Appl. Catal. B-Environ. 201 (2017) 128-136. DOI:10.1016/j.apcatb.2016.08.030 |
| [12] |
J.Y. Huang, S.H. Li, M.Z. Ge, et al., J. Mater. Chem. A Mater. Energy Sustain. 3 (2015) 2825-2832. DOI:10.1039/C4TA05332J |
| [13] |
X. Li, J. Wang, Z. Hu, M. Li, K. Ogino, Chin. Chem. Lett. 29 (2018) 166-170. DOI:10.1016/j.cclet.2017.05.020 |
| [14] |
H.Y. Wang, Y. Yang, X. Li, L.J. Li, C. Wang, Chin. Chem. Lett. 21 (2010) 1119-1123. DOI:10.1016/j.cclet.2010.03.009 |
| [15] |
S. Xie, J. Zhao, B. Zhang, et al., ACS Appl. Mater. Interfaces 7 (2015) 17558-17564. DOI:10.1021/acsami.5b04231 |
| [16] |
B. Xue, Z. Zhang, Y. Sun, et al., Carbohyd. Polym. 186 (2018) 176-183. DOI:10.1016/j.carbpol.2017.12.088 |
| [17] |
M.A. Green, A. Ho-Baillie, H.J. Snaith, Nat. Photonics 8 (2014) 506-514. DOI:10.1038/nphoton.2014.134 |
| [18] |
F. Zheng, D. Saldana-Greco, S. Liu, A.M. Rappe, J. Phys. Chem. Lett. 6 (2015) 4862-4872. DOI:10.1021/acs.jpclett.5b01830 |
| [19] |
G.L. Yang, H.Z. Zhong, Chin. Chem. Lett. 27 (2016) 1124-1130. DOI:10.1016/j.cclet.2016.06.047 |
| [20] |
L.C. Schmidt, A. Pertegas, S. Gonzalez-Carrero, et al., J. Am. Chem. Soc. 136 (2014) 850-853. DOI:10.1021/ja4109209 |
| [21] |
F. Zhang, H. Zhong, C. Chen, et al., ACS Nano 9 (2015) 4533-4542. DOI:10.1021/acsnano.5b01154 |
| [22] |
L. Protesescu, S. Yakunin, M.I. Bodnarchuk, et al., Nano Lett. 15 (2015) 3692-3696. DOI:10.1021/nl5048779 |
| [23] |
A. Swarnkar, R. Chulliyil, V.K. Ravi, et al., Angew. Chem. Int. Ed. 54 (2015) 15424-15428. DOI:10.1002/anie.201508276 |
| [24] |
Y. Wang, X. Li, J. Song, et al., Adv. Mater. 27 (2015) 7101-7108. DOI:10.1002/adma.201503573 |
| [25] |
J. Song, J. Li, X. Li, et al., Adv. Mater. 27 (2015) 7162-7167. DOI:10.1002/adma.201502567 |
| [26] |
Y. Wang, X. Li, X. Zhao, et al., Nano Lett. 16 (2016) 448-453. DOI:10.1021/acs.nanolett.5b04110 |
| [27] |
S. Yakunin, L. Protesescu, F. Krieg, et al., Nat. Commun. 6 (2015) 8056.. DOI:10.1038/ncomms9056 |
| [28] |
Q.A. Akkerman, V. D'Innocenzo, S. Accornero, et al., J. Am. Chem. Soc. 137 (2015) 10276-10281. DOI:10.1021/jacs.5b05602 |
| [29] |
G. Nedelcu, L. Protesescu, S. Yakunin, et al., Nano Lett. 15 (2015) 5635-5640. DOI:10.1021/acs.nanolett.5b02404 |
| [30] |
F. Palazon, Q.A. Akkerman, M. Prato, L. Manna, ACS Nano 10 (2016) 1224-1230. DOI:10.1021/acsnano.5b06536 |
| [31] |
G. Grancini, V. D'Innocenzo, E.R. Dohner, et al., Chem. Sci. 6 (2015) 7305-7310. DOI:10.1039/C5SC02542G |
| [32] |
J.F. Galisteo-Lopez, M. Anaya, M.E. Calvo, H. Miguez, J. Phys. Chem. Lett. 6 (2015) 2200-2205. DOI:10.1021/acs.jpclett.5b00785 |
| [33] |
A.M.A. Leguy, Y. Hu, M. Campoy-Quiles, et al., Chem. Mater. 27 (2015) 3397-3407. DOI:10.1021/acs.chemmater.5b00660 |
| [34] |
G.E. Eperon, S.N. Habisreutinger, T. Leijtens, et al., ACS Nano 9 (2015) 9380-9393. DOI:10.1021/acsnano.5b03626 |
 2019, Vol. 30
2019, Vol. 30 

