Cellular regulatory programs usually require the orchestration of multi-enzyme processes to carry out a consecutive series of biochemical reactions, executing various exquisite life processes such as energy conversion, biosynthesis, detoxification and signal conduction [1, 2]. To be specific, different types of enzyme are spatially confined in organelles or self-assembled into complexes [3, 4]. Such close confinement helps to entrap the intermediates on the confined multi-enzyme systems and minimize the diffusion of intermediates [3-5]. As a result, the conversion of intermediates into product is significantly accelerated, and side reactions are greatly inhibited, thus remarkably enhancing the overall efficiency and specificity [3-7]. Construction of these spatially confined multi-enzyme systems not only provides deep insight to physiological and pathological mechanisms but also is of great importance for biosynthesis, bioanalysis and biomedicine [3, 4]. Recently, a host of strategies, including liposomes encapsulating [8], polymersomes entrapping [1], chemical crosslinking [9, 10] and covalent conjugation [11, 12], have been exploited to arrange multiple enzymes into a confined space for the construction of artificial multi-enzyme complexes. Although promising, there are still several obstacles remaining which may impede the practical applications. For example, synthesis of these multi-enzyme complexes involves tedious preparations and multiple steps, leading to the loss of enzyme activity and further decreasing the catalytic efficiency [12-15]. Therefore, it remains greatly challenging to develop a simple and efficient strategy to build spatially confined multi-enzyme complexes.
Owing to their robust stability and easy synthesis, nanozymes have attracted numerous interests and been regarded as a promising alternative to natural enzymes [16-22]. Among various nanozymes, CeO2 is of particular interest because of its exceptional oxygen-storage capacity, mixed oxidation state (Ce(Ⅲ) and Ce(Ⅳ)), and high catalytic reactivity [23-27]. Through switching between Ce(Ⅲ) and Ce(Ⅳ), CeO2 exhibits strong redox behavior and display mimetic properties of multiple enzymes such as oxidase, catalase, superoxide dismutase and peroxidase [28, 29]. More significantly, the high specific surface area contributes to rich surface Ce atoms and offers numerous active sites for interaction with biomolecules, making nanoceria as a promising building block for construction of multicomponent and multifunctional biomaterials [30-33]. In this regard, self-assembly of CeO2 and natural enzymes could be a simple and highly promising strategy to construct highly efficient multi-enzyme nanocomplexes.
In this work, taking self-assembled CeO2/glucose oxidase (CeO2/GOx) as an example, we demonstrate the feasibility to co-confine CeO2 and enzymes in a restricted space to form hybrid nanozyme complexes. As shown in Scheme 1, in the hybrid nanozyme complexes, glucose oxidase can catalyze the oxidation of glucose with the simultaneous generation of H2O2, and at the same time, H2O2 can be immediately degraded by CeO2 with minimal diffusion. As a result, CeO2/GOx exhibits improved catalytic efficiency when compared to mixed GOx and CeO2 nanoparticles. In addition, the hybrid nanozyme complex displays excellent recyclability and long-term stability. Benefiting from these merits, the hybrid nanozyme complexes were utilized to construct a colorimetric glucose biosensor with high selectivity and sensitivity. Our demonstration of self-assembled CeO2/GOx hybrid nanozyme complexes offers a vivid and simple example of efficient multi-enzyme systems, holding great promise in biosensing, biosynthesis and biomedicine.

|
Download:
|
| Scheme 1. Schematic illustration of the cascade reaction catalyzed by hybrid CeO2/GOx complexes. | |
CeO2 nanoparticles were prepared according to a previously reported method with a facile hydrothermal synthesis [25]. Transmission electron microscopy (TEM) was used to investigate the structure and morphology of the obtained CeO2 nanoparticles. As shown in Fig. 1a, it can be clearly seen that the CeO2 nanoparticles distributed homogeneously with a narrow size distribution about 4.5 nm. High-resolution TEM image (Fig. S2 in Supporting information) further shows a well-defined crystalline fringe of CeO2 nanoparticles and that the interplanar spacing is 0.32 nm, corresponding to the d spacing of (111) plane. Energy dispersive spectroscopy (EDS) and X-ray diffraction (XRD) were also used to investigate the structure and composition of CeO2 nanoparticles, as presented in Figs. S4 and S7 (Supporting information), respectively. All these results indicate that CeO2 nanoparticles were successfully synthesized. Then, the obtained nanocrystals were assembled with GOx and TEM were performed to characterize the morphology of negatively-stained GOx and CeO2/GOx nanocomplexes. For TEM observation, GOx and CeO2/GOx solution was respectively dropped onto a carbon-coated copper grid and dried in air at room temperature, followed by negatively staining with 2% phosphotungstic acid. It is obvious in Fig. 1b that the GOx displays an excellent dispersity with an average size about 15 nm. The image of CeO2/GOx nanocomplexes (Fig. 1c) shows GOx are assembled to the surface of CeO2 nanoparticles, and these two build units are interacted tightly to form well-integrated nanocomplexes. The SDS-PAGE result (Fig. 1d) shows identical mobilities for GOx and CeO2/GOx nanocomplexes, further indicating the integrality of nanocomplexes. Taken all together, these results confirmed the successful assembly of CeO2/GOx nanocomposites.

|
Download:
|
| Fig. 1. TEM images of (a) CeO2, (b) glucose oxidase (negatively stained) and (c) CeO2/GOx nanozyme complexes (negatively stained). (d) SDS-PAGE gels of (1) CeO2/GOx nanozyme complexes, (2) CeO2 and (3) GOx. | |
To investigate the catalytic properties of CeO2/GOx nanozyme complexes, a classic colorimetric reaction was conducted whereby ABTS serves as a chromogenic reagent. With the cascade catalytic reaction, produced ABTS●+ exhibits a strong absorbance at 420 nm. As is shown in Fig. 2a, when there is only GOx alone in the solution, the reaction system shows no obvious color change as well as negligible absorbance variation at 420 nm. Similar results were obtained for the solution containing only CeO2 nanoparticles, indicating that only GOx or CeO2 nanoparticles alone cannot catalyze this cascade reaction. In the presence of CeO2/GOx nanozyme complexes, the reaction solution containing CeO2/GOx nanocomposites displays a remarkable absorbance variation, and the color of the solution system quickly changes to deep green in a few minutes. Even though the solution containing mixed CeO2 and GOx also displays an absorbance variation at 420 nm, the intensity change is much lower than that of the system containing nanocomplexes, indicating that the catalytic activity of CeO2/GOx nanozyme complexes is much higher than that of mixed CeO2 and GOx. Furthermore, time-dependent absorption intensity changes at 420 nm were measured to record the cascade reaction process. As presented in Fig. 2b, the absorption intensity of the solution containing CeO2/GOx nanozyme complexes increases much faster than that of the solution containing mixed CeO2 and GOx, indicating that the nanocomplexes exhibit a faster reaction rate compared to mixtures. A similar result was obtained when timedependent absorbance spectra was analyzed and compared, as shown in Figs. 2c and d. The fast reaction rate of nanocomplexes can be ascribed to nanoscale spatial confinement of intermediates. To be specific, for the CeO2/GOx nanozyme composites, since CeO2 and GOx were binding closely together, the in situ generated H2O2 by GOx would easily react with the surrounding CeO2 nanoparticles and be degraded with minimal diffusion. While for the mixed CeO2 and GOx, the generated H2O2 needed to diffuse with a long distance to interact with CeO2. Such a long diffusion distance decreases the catalytic rate for the cascade reactions, and moreover, H2O2 is likely to undergo self-decomposition without reaction with CeO2, further decreasing the catalytic efficiency. These findings suggest that CeO2/GOx nanozyme complexes display enhanced catalytic activity to the cascade reaction.
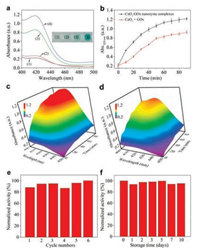
|
Download:
|
| Fig. 2. (a) UV–vis absorption spectra of the reaction systems followed by the addition of (1) CeO2 only, (2) GOx only, (3) the mixture of CeO2 and GOx, (4) CeO2/GOx nanozyme complexes. The corresponding photographs are presented in the insets. (b) Time-dependent absorbance intensity at 420 nm of the reactions solutions containing the mixture and nanozyme complexes. Time-dependent UV–vis absorption spectra of the reaction solutions containing (c) CeO2/GOx nanozyme complexes and (d) the mixture. Cascade catalytic activity of the CeO2/GOx nanocomplexes with different (e) cycle numbers and (f) storage time. | |
Stability especially long-term stability and recyclability is recognized as one of the most significant features. Enzymes with high stability can not only meet the requirements of practical use but also ameliorate the process economy of industrial enzymatic catalysis [11, 34]. Therefore, the multi-enzyme with high stability is of critical importance for practical applications. The recycling experiments of CeO2/GOx were carried out in the way that nanocomplexes were concentrated through centrifugation after every cycle, followed by washing twice with buffer solution and finally being redispersed for further use. As shown in Fig. 2e, only a little decrease of the catalytic activity was observed after 5 cycles. That is to say, the activity of the nanozyme composites maintained at a relatively high level after several reaction cycles. Probably, this slight decrease may be due to the loss of the nanocomplexes during centrifugation. In addition, the long-term stability CeO2/GOx was also investigated. More specifically, the nanozyme complexes were stored at about 25 ℃, and the catalytic activity to cascade reaction was measured during 10 days as presented in Fig. 2f. It can be observed that CeO2/GOx nanocomplexes exhibited high catalytic efficiency even after storage for 7 days, indicating that the CeO2/GOx nanocomplexes possess excellent long-term stability. Overall, the CeO2/GOx nanocomplexes display excellent recyclability for several times and outstanding long-time stability over an extended period, holding great promise in practical applications.
Glucose plays an important role in biosystems as the energy source and metabolic intermediate of living cells [21]. The aberrant concentration of glucose is closely related to the occurrence and development of many diseases such as high blood pressure, diabetes and cancer [35-37]. Therefore it is very important for the quantitative detection of glucose. To demonstrate the applications of CeO2/GOx nanocomplexes, a high-performance biosensor was built for the colorimetric detection of glucose with high sensitivity and selectivity. In the presence of glucose, the reaction solution exhibits a characteristic absorbance spectrum with a peak at 420 nm. Moreover, with the increased concentration of glucose, absorbance intensity at 420 nm increases gradually and shows a positive correlation to glucose concentration. Fig. 3a shows that the absorbance at 420 nm was proportional to the concentration of glucose and that the biosensor can detect glucose solution with concentration as low as 25 μmol/L. As shown in the inset, it is obviously that with the increase of glucose concentration, the reaction solution exhibits a remarkable color change from colorless to deep green, suggesting that the concentration of glucose can be read out with the naked eye. These results indicate that CeO2/GOx nanocomplexes-based biosensor can be utilized for the colorimetric detection of glucose with high sensitivity. Furthermore, the selectivity of CeO2/GOx nanocomplexes-based biosensor was tested under the same conditions using fructose, galactose, mannose, maltose, lactose and saccharose as contrast. As indicated in Fig. 3b, all of these glucose analogues generate negligible absorption variation. In contrast, there was an evident absorbance variation in the reaction system with glucose, demonstrating that the CeO2/GOx nanocomplexes-based biosensing platform also displays high selectivity towards glucose. All these results demonstrated that the developed colorimetric method exhibited high sensitivity and selectivity toward glucose, exhibiting great potential in applications such as biosensing, fundamental research and disease diagnosis.
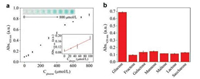
|
Download:
|
| Fig. 3. (a) Absorbance of the reactions solution at λ = 420 nm with different glucose concentration. The insets show the corresponding photographs (top left) and the initial linear response (low right). (b) Selectivity assays for CeO2/GOx-based glucose biosensors. The concentrations of glucose and its analogues are all kept at 1 mmol/L. | |
In summary, a hybrid nanozyme complex was developed through self-assembly of CeO2 nanoparticles and GOx and displayed excellent catalytic activity toward cascade reactions. CeO2 nanoparticles exhibit peroxidase-mimicking catalytic activity which can catalyze the H2O2 generated by GOx. Compared to mixed CeO2 and GOx, the nanocomplexes showed much improved catalytic efficiency because of the minimal diffusion of intermediates. Moreover, CeO2/GOx nanocomplexes also exhibited robust recycle stability and excellent long-term stability, holding great promise in practical applications. A colorimetric biosensor for glucose detection was built based on CeO2/GOx nanocomplexes, and showed high sensitivity and selectivity, holding great promise in fundamental research and diagnosis. Given the multienzyme mimicking properties of CeO2 nanoparticles and its general interaction with proteins, CeO2 can be assembled with other enzymes to various multi-enzyme nanocomplexes, leading to the development of a new family of hybrid nanozyme complexes.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (No. 21675120), National Key R & D Program of China (No. 2017YFA0208000) and Ten Thousand Talents Program for Young Talents. Q. Yuan thanks the Large-scale Instrument and Equipment Sharing Foundation of Wuhan University.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.12.021.
| [1] |
Y. Liu, J.J. Du, M. Yan, et al., Nat. Nanotechol. 8 (2013) 187-192. DOI:10.1038/nnano.2012.264 |
| [2] |
R.J. Conrado, J.D. Varner, M.P. DeLisa, Curr. Opin. Biotechnol. 19 (2008) 492-499. DOI:10.1016/j.copbio.2008.07.006 |
| [3] |
A. Küchler, M. Yoshimoto, S. Luginbühl, F. Mavelli, P. Walde, Nat. Nanotechnol. 11 (2016) 409-420. DOI:10.1038/nnano.2016.54 |
| [4] |
Z. Zhao, J.L. Fu, S. Dhakal, et al., Nat. Commun. 7 (2016) 1-9. |
| [5] |
N.N. Liu, Z.K. Yang, X.D. Lou, et al., Anal. Chem. 87 (2015) 4037-4041. DOI:10.1021/acs.analchem.5b00375 |
| [6] |
S.Z. Wang, Y.H. Zhang, H. Ren, et al., Crit. Rev. Biotechnol. 37 (2017) 1024-1037. DOI:10.1080/07388551.2017.1303803 |
| [7] |
J.J.X. Wu, S.R. Li, H. Wei, Chem. Commun. 54 (2018) 6520-6530. DOI:10.1039/C8CC01202D |
| [8] |
A. Graff, M. Winterhalter, W.G. Meier, Langmuir 17 (2001) 919-923. DOI:10.1021/la001306m |
| [9] |
H. Liang, S.H. Jiang, Q.P. Yuan, et al., Nanoscale 8 (2016) 6071-6078. DOI:10.1039/C5NR08734A |
| [10] |
Q. Wu, X. Wang, C.A. Liao, Q.C. Wei, Q.G. Wang, Nanoscale 7 (2015) 16578-16582. DOI:10.1039/C5NR05716G |
| [11] |
Y.F. Zhang, J. Ge, Z. Liu, ACS Catal. 5 (2015) 4503-4513. DOI:10.1021/acscatal.5b00996 |
| [12] |
J.L. Fu, Y.R. Yang, A. Johnson-Buck, et al., Nat. Nanotechnol. 9 (2014) 531-536. DOI:10.1038/nnano.2014.100 |
| [13] |
M.X. Chen, J. Qi, D.Y. Guo, et al., Chem. Commun. 53 (2017) 9566-9569. DOI:10.1039/C7CC05172G |
| [14] |
R.X. Duan, B.Y. Wang, F. Hong, et al., Nanoscale 7 (2015) 5719-5725. DOI:10.1039/C5NR00697J |
| [15] |
X.J. Guo, X.L. Li, X.C. Liu, et al., Chem. Commun. 54 (2018) 845-848. DOI:10.1039/C7CC09383G |
| [16] |
J.F. Shi, Y.Z. Wu, S.H. Zhang, et al., Chem. Soc. Rev. 47 (2018) 4295-4313. DOI:10.1039/C7CS00914C |
| [17] |
Y.H. Lin, J.S. Ren, X.G. Qu, Acc. Chem. Res. 47 (2014) 1097-1105. DOI:10.1021/ar400250z |
| [18] |
H. Wei, E.K. Wang, Chem. Soc. Rev. 42 (2013) 6060-6093. DOI:10.1039/c3cs35486e |
| [19] |
A. Asati, S. Santra, C. Kaittanis, S. Nath, J.M. Perez, Angew. Chem. Int. Ed. 121 (2009) 2344-2348. DOI:10.1002/ange.v121:13 |
| [20] |
L.Z. Gao, J. Zhuang, L. Nie, et al., Nat. Nanotechnol. 2 (2007) 577-583. DOI:10.1038/nnano.2007.260 |
| [21] |
Z.H. Li, X.D. Yang, Y.B. Yang, et al., Chem.-Eur. J. 24 (2018) 409-415. DOI:10.1002/chem.v24.2 |
| [22] |
Y.J. Guo, L. Deng, J. Li, et al., ACS Nano 5 (2011) 1282-1290. DOI:10.1021/nn1029586 |
| [23] |
X. Wang, Y.B. Zhang, S.Y. Song, et al., Angew. Chem. Int. Ed. 128 (2016) 4618-4622. DOI:10.1002/ange.201600625 |
| [24] |
Z.E. Liu, J. Wang, Y. Li, et al., ACS Appl. Mater. Interfaces 7 (2015) 19416-19423. DOI:10.1021/acsami.5b05633 |
| [25] |
X.Y. Liu, W. Wei, Q. Yuan, et al., Chem. Commun. 48 (2012) 3155-3157. DOI:10.1039/C1CC15815E |
| [26] |
Y.Q. Ge, P.H. Diao, C. Xu, N.N. Zhang, C. Guo, Chin. Chem. Lett. 29 (2018) 903-906. DOI:10.1016/j.cclet.2018.01.002 |
| [27] |
Y.N. Men, J. Su, X.L. Wang, et al., Chin. Chem. Lett. 29 (2018) 903-906. DOI:10.1016/j.cclet.2018.01.002 |
| [28] |
B.W. Liu, Z.Y. Sun, P.J. Huang, J.W. Liu, J. Am. Chem. Soc. 137 (2015) 1290-1295. DOI:10.1021/ja511444e |
| [29] |
H. Zhao, Y.M. Dong, P.P. Jiang, G.L. Wang, J.J. Zhang, ACS Appl. Mater. Interfaces 7 (2015) 19416-19423. DOI:10.1021/acsami.5b05633 |
| [30] |
Y.H. Lin, C. Xu, J.S. Ren, X.G. Qu, Angew. Chem. Int. Ed. 124 (2012) 12747-12751. DOI:10.1002/ange.201207587 |
| [31] |
Y. Long, S. Song, J. Li, et al., ACS Catal. 8 (2018) 8506-8512. DOI:10.1021/acscatal.8b01851 |
| [32] |
X.M. Wang, X.M. Wang, P.F. Huang, et al., Talanta 181 (2018) 112-117. DOI:10.1016/j.talanta.2018.01.004 |
| [33] |
S.S. Zhou, H.J. Wang, P.X. Jin, et al., J. Chromatogr. A 1569 (2018) 17-25. DOI:10.1016/j.chroma.2018.07.052 |
| [34] |
X. Lu, S. He, W. Cheng, J. Shi, Chin. Chem. Lett. 29 (2018) 1001-1008. DOI:10.1016/j.cclet.2018.05.011 |
| [35] |
G.B. Mao, Q. Cai, F.B. Wang, et al., Anal. Chem. 89 (2017) 11628-11635. DOI:10.1021/acs.analchem.7b03053 |
| [36] |
W.T. Wu, T. Zhou, A. Berliner, P. Banerjee, S.Q. Zhou, Angew. Chem. Int. Ed. 49 (2010) 6554-6558. DOI:10.1002/anie.201001508 |
| [37] |
B. Hai, Y. Zou, G. Guo, et al., Chin. Chem. Lett. 28 (2017) 149-152. DOI:10.1016/j.cclet.2016.07.023 |
 2019, Vol. 30
2019, Vol. 30 

