Nitriles are versatile important organic synthesis intermediates in organic chemistry. They can be easily transformed into amides [1, 2], amines [3, 4], ketones [5, 6], carbonic acids [7, 8], esters [9] and other important functional group [10-13]. Furthermore, the cyano group, as a key pharmacophore, widely exists in the structure of many bioactive compounds and drugs (Scheme 1) [14-18]. Classical synthetic methods of nitriles are Sandmeyer reaction [19] and Rosenmund-von Braun reaction [20] which require highly toxic cyanide salts and produce large amounts of inorganic salts as waste. Also some metal-catalyzed oxidative synthesis of nitriles from primary amines or alcohols have been developed and achieved great success [21, 22]. Direct synthesis of nitriles from aldehydes via the Schmidt reaction was a promising way [23-25], while it has not received wide acceptance by the synthetic community likely due to the use of highly toxic and dangerous azide compounds. Recently, Rokade and Prabhu [26] reported that NaN3/TfOH provides a superior medium for selective conversion of aromatic aldehydes to nitriles, whereas it still produces the toxic HN3 and excessive amounts of TfOH was needed (Scheme 2a). Laali [27] has also successfully achieved the selective Schmidt reaction of aldehydes in ionic liquids (Scheme 2b).
|
Download:
|
| Scheme 1. Nitriles in bioactive natural products, pharmaceuticals and pesticide. | |

|
Download:
|
| Scheme 2. Selective Schmidt reaction. | |
In the past decade, micro-reactor technology has emerged as a powerful tool for solving synthetic problems in the field of pharmaceutical and fine chemical research due to its environmental friendliness, efficiency and safety [28-36]. The recent special issue in Bioorganic & Medicinal Chemistry has reported the organic synthesis in flow for medicinal chemistry [37]. The azides have been successfully applied in several reactions in continuous flow mode [38-41]. For example, Yanada and coworkers developed an efficient one-pot synthesis of 1, 2, 3-triazole-fused isoindoles from o-alkynylbenzaldehydes and trimethylsilyl azide (TMSN3) through the formation of a diazide intermediate and a subsequent intramolecular azide-alkyne cycloaddition in high yields [42]. Yakambram reported a simple and convenient metal free procedure for the synthesis of 5-substituted 1H-tetrazoles using various nitriles and sodium azide in the presence of urea and acetic acid with good to high yields [43]. A microreactor setup was developed in which methyl azide (MeN3) was successfully generated and performed, demonstrating that the flow method serves as a safe method for handling hazardous explosive methyl azide [44].
Therefore, we envisioned to synthesize nitriles from aldehydes via the classical Schmidt reaction in flow mode in order to improve the safety and make it easy to scale up.
In this experiment, 1H and 13C NMR spectra were recorded on Bruker NMR spectrometer (1H 400 MHz, 13C 100 MHz). HPLC analysis was performed on Agilent 1260 (DAD: 254 nm; column: agilent zorbax eclipse ZORBAX SB-C18 (4.6 mm × 150 mm, I.D. 5 μm); mobile phase: MeCN-water, 30% MeCN within 20 min, flow rate 1.0 mL/min). Selective hydrolysis of nitriles was performed in a Labtrix S1 continuous flow system equipped with a SOR-mixer reactor 3222 (Width channel: 300 μm, Depth channel: 120 μm, Reactor Volume: 5 μL) and a back pressure regulator (0–20 bar). The pictures of Labtrix S1 and SOR-mixer reactor 3222 are shown in Supporting information. Syringes were purchased from SGE Analytical Science (I.D. = 4.610 mm, volume = 1 mL).
General procedure for synthesis of nitriles from aldehydes in continuous flow is as follows. Take benzaldehyde as an example, benzaldehyde (0.6 mmol) and TfOH (0.9 mmol) were dissolved in 3 mL MeCN and pumped into inlet A. TMSN3 (0.6 mmol) was dissolved in 3 mL MeCN and pumped into inlet B (flow rate A: B = 54 μL/min:65 μL/min for 5 s residence time). The whole system was maintained at 25 ℃. The flow system was equilibrated (about 5 min), then the product stream was quenched and collected in a glass vessel with saturated aqueous NaClO in it. The flow system was stopped after the inlet A reduced 0.5 mL. The crude mixture was dissolved in ethyl acetate and washed with saline solution. The combined organic layers were dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The resulting crude product was purified by flash chromatography on silica gel with hexane and EtOAc as eluent to afford the product (details seeing Supporting information).
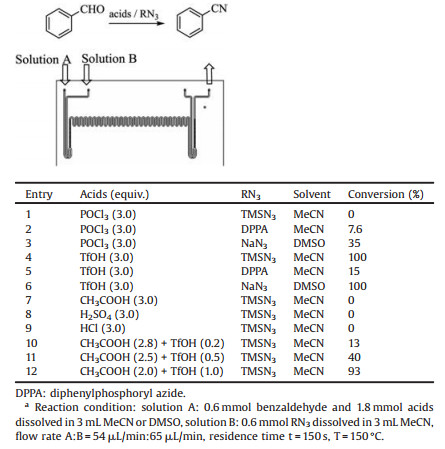
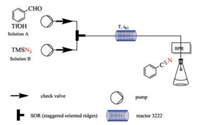
Firstly, benzaldehyde was chosen as a model reaction substrate, acetonitrile as solvent, different acids and azides were tested in microreactors. As shown in Table 1, NaN3 and TMSN3 were both capable for conducting this reaction in the presence of TfOH. Comparing to TMSN3, NaN3 is more toxic and need DMSO as solvent, so TMSN3 was selected to the following screening. Considering TfOH is costly, a mixed acids of TfOH and acetic acid with different ratio were also examined and the conversion ratio was not good enough (Table 1, entries 10–12). Hence, TMSN3 and TfOH was chosen to establish the following flow-platform: Benzaldehyde and TfOH were taken up in MeCN and pumped into inlet A, while TMSN3 was pumped into inlet B. The reaction stream was pressurized by a 10 bar back pressure regulator (BPR) and the outflow was collected. After being filtered, the outflow was analyzed by HPLC to determine the conversion (Scheme 3).
|
Download:
|
| Scheme 3. A simple set-up for flow platform. | |
|
|
Table 1 Different combination of acids and azides.a |
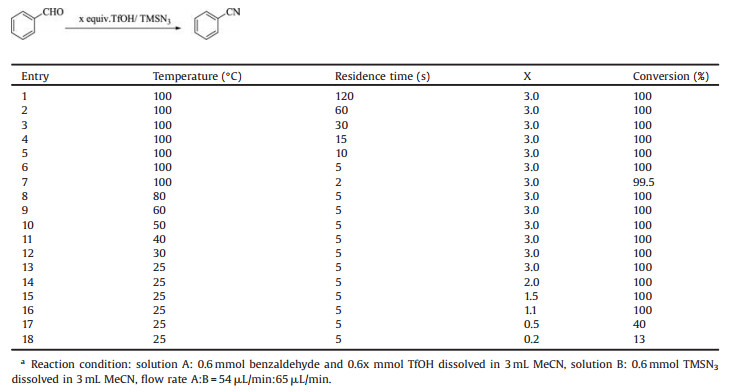
Next, we turned our attention to the optimization of the reaction parameters in a continuous-flow microreactor (Table 2). It can be seen that the conversion ratio was quantitative even if the residence time decreased from 120 s to 5 s (Table 2, entries 1–7). The change of temperature did not affect the reaction, either. The conversion rate of the reaction maintained 100% when the reaction temperature dropped from 100 ℃ to 25 ℃ (Table 2, entries 6, 8– 13). Reducing the amount of TfOH to 1.1 equiv. would not cause any loss of conversion (Table 2, entries 13–18). Taking into account the difference of substrate activity and some follow-up exploratory experiments, we confirmed that the final response parameters were the reaction temperature of 25 ℃, the residence time of 5–15 s and the TfOH of 1.5 equiv.
|
|
Table 2 Optimization of residence time, temperature and the equivalent of TfOH.a |
With the optimized reaction conditions in hand, we next surveyed the scope of this approach. As shown in Scheme 4, all tested substrates produced the expected nitriles in good to excellent yields. For substituted benzaldehydes, whatever it bearing electron-donating or electron-withdrawing groups, i.e., halogen, methoxyl, trifluoromethyl, amino and nitro, were all tolerated in this transformation (1b-1g, 88%–95%). The position of substituent had little effect on the reaction (1g-1l). The substrates bearing aryl substitution or polysubstitution could also react well to provide the desired nitriles as long as the residence time was prolonged to 10 s (1m-1n). The presence of extra functional group such as hydroxyl or cyano on benzaldehydes had no effect on the reaction (1o-1p). Heteroaromatic aldehydes were suitable as well, leading to the corresponding products 1q-1s in excellent yields. Notably, phenyl acetaldehyde and cinnamaldehyde could also be smoothly transformed into the desired nitriles 1t and 1u in 98% and 88% yields, respectively.
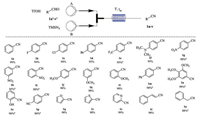
|
Download:
|
| Scheme 4. Substrate scope under optimized condition. Reaction condition: solution A: 0.6 mmol substituted benzaldehyde and 0.9 mmol TfOH dissolved in 3 mL MeCN, solution B: 0.6 mmol TMSN3 dissolved in 3 mL MeCN. Flow rate A:B = 54 μL/min:65 μL/min, residence time t = 5 s, T = 25 ℃. aResidence time is 10 s | |
In summary, we have successfully carried out the synthesis of nitriles via Schmidt reaction in a continuous-flow microreactor. The advantages of this method include broad substrate scope, high yields, rapid reaction, simplicity and convenience. Most importantly, this procedure greatly improves the safety and is easy to scale up. Thus, the approach will be very useful in the preparation of various nitriles.
AcknowledgmentThe authors thank the National Natural Science Foundation of China (No. 21877005) for financial support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.01.006.
| [1] |
A.R. Katritzky, B. Pilarski, L. Urogdi, Synthesis 1989 (1989) 949-950. DOI:10.1055/s-1989-27441 |
| [2] |
J. Borau-Garcia, D.V. Gutsulyak, R.J. Burford, W.E. Piers, Dalton Trans. 44 (2015) 12082-12085. DOI:10.1039/C4DT03902E |
| [3] |
F. Gould, G. Johnson, A. Ferris, J. Org. Chem. 25 (1960) 1658-1660. |
| [4] |
H. Adkins, H.R. Billica, J. Am. Chem. Soc. 70 (1948) 695-698. DOI:10.1021/ja01182a080 |
| [5] |
W.B. Jang, W.S. Shin, J.E. Hong, S.Y. Lee, D.Y. Oh, Synth. Commun. 27 (2006) 3333-3339. |
| [6] |
M. Ortiz-Marciales, L.M. Tirado, R. Colón, M.L. Ufret, R. Figueroa, M. Lebrón, M. DeJesús, J. Martínez, T. Malavé, Synth. Commun. 28 (1998) 4067-4075. DOI:10.1080/00397919808004967 |
| [7] |
D. Brady, N. Dube, R.S. Petersen, Afr. J. Sci. 102 (2006) 339-344. |
| [8] |
J. Raj, N. Singh, S. Prasad, A. Seth, T.C. Bhalla, Acta Microbiol. Immunol. Hung. 54 (2007) 79-88. DOI:10.1556/AMicr.54.2007.1.8 |
| [9] |
M. Noè, A. Perosa, M. Selva, Green Chem. 15 (2013) 2252-2260. DOI:10.1039/c3gc40774h |
| [10] |
W.J. Qi, Y. Han, C.Z. Liu, C.G. Yan, Chin. Chem. Lett. 28 (2017) 442-445. DOI:10.1016/j.cclet.2016.09.014 |
| [11] |
Z.H. Weng, Y. Qi, L.S. Zong, et al., Chin. Chem. Lett. 28 (2017) 1069-1073. DOI:10.1016/j.cclet.2016.12.019 |
| [12] |
L.Y. Xie, S. Peng, L.H. Lu, et al., ACS Sustainable Chem. Eng. 6 (2018) 7989-7984. DOI:10.1021/acssuschemeng.8b01358 |
| [13] |
L.Y. Xie, S. Peng, F. Liu, et al., Adv. Synth. Catal. 360 (2018) 4259-4264. DOI:10.1002/adsc.v360.21 |
| [14] |
F.F. Fleming, L. Yao, P.C. Ravikumar, L. Funk, B.C. Shook, J. Med. Chem. 53 (2010) 7902-7917. DOI:10.1021/jm100762r |
| [15] |
M. Frizler, F. Lohr, N. Furtmann, J. Klas, M. Gutschow, J. Med. Chem. 54 (2011) 396-400. DOI:10.1021/jm101272p |
| [16] |
C.P. Chuck, C. Chen, Z. Ke, D.C. Wan, H.F. Chow, K.B. Wong, Eur. J. Med. Chem. 59 (2013) 1-6. DOI:10.1016/j.ejmech.2012.10.053 |
| [17] |
H.B. Diane, Y. Fei, D.W. Yanong, et al., J. Med. Chem. 44 (2001) 3965-3977. DOI:10.1021/jm0102250 |
| [18] |
L. Else, V. Watson, J. Tjia, et al., J. Chromatogr. B. 878 (2010) 1455-1465. DOI:10.1016/j.jchromb.2010.03.036 |
| [19] |
D.T. Mowry, Chem. Rev. 42 (1948) 189-283. DOI:10.1021/cr60132a001 |
| [20] |
G.P. Ellis, T.M. Romney-Alexander, Chem. Rev. 87 (1987) 779-794. DOI:10.1021/cr00080a006 |
| [21] |
X.T. Ma, H. Xu, Y.L. Xiao, et al., Chin. Chem. Lett. 28 (2017) 1336-1339. DOI:10.1016/j.cclet.2017.01.017 |
| [22] |
Y.K. Hu, L. Chen, B.D. Li, Chin. Chem. Lett. 29 (2018) 464-466. DOI:10.1016/j.cclet.2017.10.036 |
| [23] |
K.F.Z. Schmidt, Angew. Chem. Int. Ed. 36 (1923) 511. |
| [24] |
K.F. Schmidt, Chem. Ber. 57 (1924) 704-706. DOI:10.1002/cber.19240570423 |
| [25] |
G.I. Koldobskii, V.A. Ostrovskii, B.V. Gidaspov, Russ. Chem. Rev. 47 (1978) 1084-1094. DOI:10.1070/RC1978v047n11ABEH002294 |
| [26] |
B.V. Rokade, K.R. Prabhu, J. Org. Chem. 77 (2012) 5364-5370. DOI:10.1021/jo3008258 |
| [27] |
G.C. Nandi, K.K. Laali, Tetrahedron Lett. 54 (2013) 2177-2179. DOI:10.1016/j.tetlet.2013.02.051 |
| [28] |
S. Roesner, S.L. Buchwald, Angew. Chem. Int. Ed. 55 (2016) 10463-10467. DOI:10.1002/anie.201605584 |
| [29] |
R. Gérardy, N. Emmanuel, T. Toupy, et al., Eur. J. Org. Chem. 2018 (2018) 2301-2351. DOI:10.1002/ejoc.201800149 |
| [30] |
R.E. Ziegler, B.K. Desai, J.A. Jee, et al., Angew. Chem. Int. Ed. 57 (2018) 7181-7185. DOI:10.1002/anie.v57.24 |
| [31] |
I.K. Alsulami, T.M.D. Alharbi, D.P. Harvey, C.T. Gibson, C.L. Raston, Chem. Commun. 54 (2018) 7896-7899. DOI:10.1039/C8CC03730B |
| [32] |
C. Battilocchio, F. Feist, A. Hafner, et al., Nat. Chem. 8 (2016) 360-367. DOI:10.1038/nchem.2439 |
| [33] |
H. Seo, M.H. Katcher, T.F. Jamison, Nat. Chem. 9 (2017) 453-456. DOI:10.1038/nchem.2690 |
| [34] |
S. Watanabe, N. Nakaya, J. Akai, K. Kanaori, T. Harada, Org. Lett. 20 (2018) 2737-2740. DOI:10.1021/acs.orglett.8b00945 |
| [35] |
P. Rulliere, G. Benoit, E.M.D. Allouche, A.B. Charette, Angew. Chem. Int. Ed. 57 (2018) 5777-5782. DOI:10.1002/anie.201802092 |
| [36] |
M.R. Chapman, S.C. Cosgrove, N.J. Turner, N. Kapur, A.J. Blacker, Angew. Chem. Int. Ed. 57 (2018) 1-6. DOI:10.1002/anie.201712460 |
| [37] |
T. Wirth, Bioorg. Med. Chem. 25 (2017) 6179. DOI:10.1016/j.bmc.2017.11.013 |
| [38] |
M. Movsisyan, E.I. Delbeke, J.K. Berton, et al., Chem. Soc. Rev. 45 (2016) 4892-4928. DOI:10.1039/C5CS00902B |
| [39] |
S. Pan, S. Yan, T. Osako, Y. Uozumi, ACS Sustain. Chem. Eng. 5 (2017) 10722-10734. DOI:10.1021/acssuschemeng.7b02646 |
| [40] |
Y. Liao, Q. Lu, G. Chen, et al., ACS Catal. 7 (2017) 7529-7534. DOI:10.1021/acscatal.7b02558 |
| [41] |
S.C. Born, C.E.R. Edwards, B. Martin, K.F. Jensen, Tetrahedron 74 (2018) 3137-3142. DOI:10.1016/j.tet.2018.01.026 |
| [42] |
N. Okamoto, T. Sueda, H. Minami, R. Yanada, Tetrahedron Lett. 59 (2018) 1461-1464. DOI:10.1016/j.tetlet.2018.03.002 |
| [43] |
B. Yakambram, A. Jaya Shree, L. Srinivasula Reddy, et al., Tetrahedron Lett. 59 (2018) 445-449. DOI:10.1016/j.tetlet.2017.12.067 |
| [44] |
D. Ichinari, A. Nagaki, J.I. Yoshida, Bioorg. Med. Chem. 25 (2017) 6224-6228. DOI:10.1016/j.bmc.2017.07.005 |
 2019, Vol. 30
2019, Vol. 30