b National Centre for Nanoscience and Technology, Beijing 100190, China
Helical architecture is one of the most interesting structures in nature. In biological system, helical structure is ubiquitous, for example double-helix DNA and α-helical protein [1-5], both dominate diverse physiological active function in the organism. Inspired by bio-system helix, various artificial helical architectures, such as helical polymers, self-assembled helical nanostructures and organic-inorganic hybrid helical nanomaterials, have been fabricated successfully [6-8]. In recent years, increasing interest has extended to apply natural and artificial helical structures in the field of chiral recognition, chiral separation and circularly polarized luminescence [9-22]. In particular, helical architectures for asymmetric catalysis have attracted a lot of attentions, for instance, double helix DNA, helical polymer and self-assembled helical nanotubes have been utilized as chiral scaffolds for asymmetric catalysis [23-34]. As a matter of fact, various natural products have been found to form helical nanostructures via selfassembly, however, little has been employed for asymmetric catalysis [35-37]. Therefore, it is highly desirable to explore enantioselective catalytic performance of natural helical assembly.
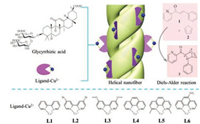
Glycyrrhizic acid (GA) is a traditional Chinese medicinal herbal, which has been applied for the treatment of liver diseases [38]. Recently, it was reported that GA could self-assemble into right handed helical nanofiber in water [39]. Herein, we tried to utilize GA based helical nanostructures as scaffolds for asymmetric catalysis. The catalyst was prepared by self-assembly of the achiral copper(Ⅱ)-ligand with GA (named as GA-L, Fig. 1). And we found that helical nanostructure were well preserved if the molar ratio of copper(Ⅱ) species to GA around a proper scope. GA-L assemblies gave moderated enantioselectivity toward Diels-Alder reaction and up to 61% ee was obtained.
|
Download:
|
| Fig. 1. Molecular structure of GA and catalyst by co-assembly of GA with copper(Ⅱ)-ligand (L1-L6), Diels-Alder reaction was selected as a model reaction. | |
GA-L was constructed via co-assembly of the copper(Ⅱ) ions coordinated ligands (L1-L6) and GA. GA was first heated to dissolve in water (1 wt%). Upon cooling, copper(Ⅱ)-ligands in water solution were injected into GA solution and mixed by shaking, the resulted mixture solution was further stand at room temperature for about 12 h.
The morphology of GA-L assembly was investigated by AFM (Atomic Force Microscope). L6 was selected as a mode ligand, and we found helical nanofibers were well preserved when the L6 amount is below 5% (molar ratio to GA). In addition, the homogenous transparent bright yellow hydrogel was formed (Figs. 2a and b). Further increase the amount of L6, the hydrogel would be destroyed and turned into yellow precipitate, in which two nanostructures of nanofiber and amorphous nanostructures were observed (Figs. 2c and d), indicating some extra of L6 were not incorporated into helical nanofibers.

|
Download:
|
| Fig. 2. The morphology of GA-L6 assembly in water, GA concentration was set as 1 wt% in the water. AFM images of GA-L6 the assembly with the molar ratio of L6 to GA: a) 1%, b) 5%, c) 10%, d) 20%. The inserted pictures in all the figures were the optical photographs of assembly in the water. Scale bars: 200 nm. | |
UV-vis and CD spectra were monitored in order to investigate the supramolecular chirality of GA-L6 assembly. GA hydrogel have no CD signal between 320 nm–450 nm (Fig. 3, dotted line), while a negative cotton effect at 352 nm was observed for GA-L6 hydrogel, which corresponded to the spectroscopic absorbance of phenanthroline skeleton (Fig. 3, solid line), indicating that achiral L6 express induced chirality due to chiral alignment onto helical nanofiber assembly.

|
Download:
|
| Fig. 3. UV–vis and CD spectra of GA (dotted line) and GA-L6 (solid line) assemblies, 5% of L6 to GA (molar ratio) was used. | |
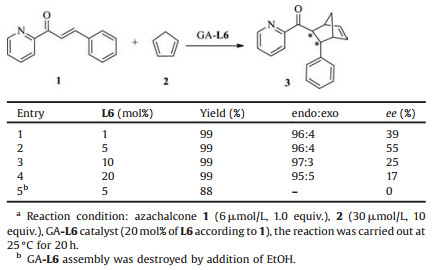
Previously, helical DNA, protein and self-assembled nanotubes have been utilized as chiral scaffolds for asymmetric catalysis after interacting with cooper(Ⅱ) complex [23-25]. In these cases, DielsAlder reaction was usually selected as benchmark reaction. Here, for exploring the catalytic behavior of GA-L assemblies, Diels-Alder reaction was also selected as a model reaction with azachalcone (1) and cyclopentadiene (2) as substrates. GA-L6 was selected as a model assembly for catalyzing Diels-Alder reaction. Due to the helical assembled morphology was related to molar ratio of L6 to GA, the catalytic behavior under various molar amount of L6 to GA was first investigated, and the results were listed in Table 1. It was found when 1 mol% of L6 was employed to co-assemble with GA, 39% ee was obtained (Table 1, entry 1), the ee value was increased to 55% when the 5 mol% L6 was used (entry 2), the ee value decreased when 10 mol% and 20 mol% L6 was co-assembled with GA (entries 3 and 4). It seemed that the homogenous helical assembly provided the high enantioselectivity. A control experiment was carried out to clarify the key role of helical structures. The addition of ethanol caused the destroying of helical architecture of GA-L6 (Fig. S1 in Supporting information), correspondingly, the resulted dispersion has no CD signal (Fig. S2 in Supporting information) and only gave racemic product (entry 5). These results indicated that self-assembled helical nanostructure was the decisive factor for offering enantioselectivity.
|
|
Table 1 GA-L-assembly for catalysis of the Diels-Alder reactiona. |
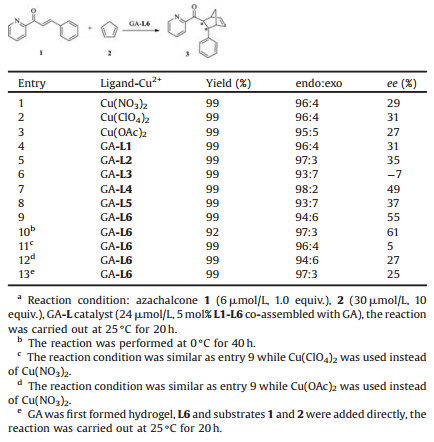
The ligand effect for the reaction was further investigated, the results were shown in Table 2. It was found that 27%–31% ee values were obtained when Cu(NO3)2, Cu(ClO4)2 and Cu(OAc)2 were directly served as copper(Ⅱ) complex (Table 2, entries 1–3), while bipyridine based assembly GA-L1 gave 31% ee (entry 4), and the enantioselectivity slight increased when brominated bipyridine based assembly GA-L2 was used as catalyst (entry 5). To our surprise, the opposite enantioselectivity was obtained when methoxyl substituted bipyridine co-assembled with GA (GA-L3, entry 6), although the low ee value (7% ee) was obtained. The 49% ee was achieved in the case of phenanthroline based ligand (GA-L4, entry 7). The enantioselectivity decreased when methyl substituted phenanthroline was used (GA-L5, entry 8). Careful optimal the reaction condition in the case of GA-L6, the ee value was improved from 55% to 61% (entries 9 and 10). Changing the ligand coordinated copper(Ⅱ) ions led to decrease enantioselectivity (entries 11 and 12). In addition, when GA was first formed hydrogel, L6 and substrates were directly added to perform the reaction, the ee value was also decreased (entry 13). These results proved that ligands and assembled condition could tune the enantioselectivity of the reaction.
|
|
Table 2 Different ligands co-assembled with GA for catalysis of the Diels-Alder reaction.a |
The probably catalytic mechanism was proposed. In GA-L assemblies, the copper (Ⅱ) coordinated ligands co-assembled with GA to form helical nanostructures. In spite of copper(Ⅱ)-ligand was achiral, a chiral microenvironment was created around the copper(Ⅱ) catalytic sites, thus the enantioselectivity for the reaction can be obtained.
In summary, glycyrrhizic acid was employed to construct supramolecular chiral catalyst toward enantioselective Diels-Alder reaction. Glycyrrhizic acid was co-assembled with achiral ligandcoordinated copper(Ⅱ), and found that the helical nanostructure could be well preserved after controlling the amount copper(Ⅱ) species to GA. The moderated enantioselectivity was obtained, and up to 61% ee was reached in the case of GA-L6 assembly. It was proved that natural product GA based self-assembled helical nanostructures could serve as chiral scaffolds for asymmetric catalysis, and much more chiral supramolecular assemblies are expected to apply in asymmetric catalysis.
AcknowledgmentsThe authors thank the National Natural Science Foundation of China (Nos. 21861132002, 21773043) and Chinese Academy of Sciences (Nos. XDB12020200, QYZDJ-SSW-SLH044) for the financial support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.02.015.
| [1] |
J.D. Watson, F.H.C. Crick, Nature 171 (1953) 737-738. DOI:10.1038/171737a0 |
| [2] |
J.C. Wang, Proc. Natl. Acad. Sci. U. S. A. 76 (1979) 200-203. DOI:10.1073/pnas.76.1.200 |
| [3] |
C.O. Pabo, R.T. Sauer, Annu. Rev. Biochem. 53 (1984) 293-321. DOI:10.1146/annurev.bi.53.070184.001453 |
| [4] |
L. Pauling, R.B. Corey, H.R. Branson, Proc. Natl. Acad. Sci. U. S. A. 37 (1951) 205-211. DOI:10.1073/pnas.37.4.205 |
| [5] |
W.G.J. Hol, P.T. Vanduijnen, H.J.C. Berendsen, Nature 273 (1978) 443-446. DOI:10.1038/273443a0 |
| [6] |
E. Yashima, N. Ousaka, D. Taura, et al., Chem. Rev. 116 (2016) 13752-13990. DOI:10.1021/acs.chemrev.6b00354 |
| [7] |
M. Liu, L. Zhang, T. Wang, Chem. Rev. 115 (2015) 7304-7397. DOI:10.1021/cr500671p |
| [8] |
L. Han, S. Che, Chem. Soc. Rev. 42 (2013) 3740-3752. DOI:10.1039/C2CS35297D |
| [9] |
T. Tu, W. Fang, X. Bao, X. Li, H.D. Karl, Angew. Chem. Int. Ed. 50 (2011) 6601-6605. DOI:10.1002/anie.v50.29 |
| [10] |
Q. Wang, J. Huang, Z.Q. Jiang, et al., Polymer 136 (2018) 92-100. DOI:10.1016/j.polymer.2017.12.057 |
| [11] |
X. Du, J. Liu, J. Deng, W. Yang, Poly. Chem. 1 (2010) 1030-1038. DOI:10.1039/c0py00028k |
| [12] |
Y. Li, P. Duan, M. Liu, Chem.-Eur. J. 23 (2017) 8225-8231. DOI:10.1002/chem.v23.34 |
| [13] |
L. Zhang, Q. Jin, M. Liu, Chem.-Asian J. 11 (2016) 2642-2649. DOI:10.1002/asia.201600441 |
| [14] |
Y. Jiang, T. Wang, M. Bai, J. Jiang, Dyes Pigments 157 (2018) 133-139. DOI:10.1016/j.dyepig.2018.04.056 |
| [15] |
J. Shen, F. Wang, Q. Bi, et al., J. Chromatogr. A (2018) 54-61. |
| [16] |
G. Li, J. Shen, Q. Li, Y. Okamoto, Chirality 27 (2015) 518-522. DOI:10.1002/chir.v27.8 |
| [17] |
W. Edwards, D.K. Smith, J. Am. Chem. Soc. 136 (2014) 1116-1124. DOI:10.1021/ja411724r |
| [18] |
K. Shimomura, T. Ikai, S. Kanoh, E. Yashima, K. Maeda, Nat. Chem. 6 (2014) 429-434. DOI:10.1038/nchem.1916 |
| [19] |
J. Shen, Y. Okamoto, Chem. Rev. 116 (2016) 1094-1138. DOI:10.1021/acs.chemrev.5b00317 |
| [20] |
Y. Nagata, K. Takagi, M. Suginome, J. Am. Chem. Soc. 136 (2014) 9858-9861. DOI:10.1021/ja504808r |
| [21] |
D. Yang, P. Duan, L. Zhang, M. Liu, Nat. Commun. 8 (2017) 15727. DOI:10.1038/ncomms15727 |
| [22] |
R. Aoki, R. Toyoda, J.F. Koegel, et al., J. Am. Chem. Soc. 139 (2017) 16024-16027. DOI:10.1021/jacs.7b07077 |
| [23] |
G. Roelfes, B.L. Feringa, Angew. Chem. Int. Ed. 44 (2005) 3230-3232. DOI:10.1002/(ISSN)1521-3773 |
| [24] |
C. Wang, G. Jia, J. Zhou, et al., Angew. Chem. Int. Ed. 51 (2012) 9352-9355. DOI:10.1002/anie.201204850 |
| [25] |
D. Coquiere, B.L. Feringa, G. Roelfes, Angew. Chem. Int. Ed. 46 (2007) 9308-9311. DOI:10.1002/(ISSN)1521-3773 |
| [26] |
A.J. Boersma, B.L. Feringa, G. Roelfes, Angew. Chem. Int. Ed. 48 (2009) 3346-3348. DOI:10.1002/anie.v48:18 |
| [27] |
Y. Li, C. Wang, G. Jia, S. Lu, C. Li, Tetrahedron 69 (2013) 6585-6590. DOI:10.1016/j.tet.2013.05.133 |
| [28] |
Z. Tang, H. Iida, H.Y. Hu, E. Yashima, ACS. Macro. Lett. 1 (2012) 261-265. DOI:10.1021/mz200161s |
| [29] |
M. Reggelin, M. Schultz, M. Holbach, Angew. Chem. Int. Ed. 41 (2002) 1614-1617. DOI:10.1002/1521-3773(20020503)41:9<>1.0.CO;2-P |
| [30] |
T. Miyabe, Y. Hase, H. Iida, K. Maeda, E. Yashima, Chirality 21 (2009) 44-50. DOI:10.1002/chir.v21:1 |
| [31] |
D. Zhang, C. Ren, W. Yang, J. Deng, Macromol. Rapid Commun. 33 (2012) 652-657. DOI:10.1002/marc.201100826 |
| [32] |
Q. Jin, L. Zhang, H. Cao, et al., Langmuir 27 (2011) 13847-13853. DOI:10.1021/la203110z |
| [33] |
J. Jiang, Y. Meng, L. Zhang, M.H. Liu, J. Am. Chem. Soc. 138 (2016) 15629-15635. DOI:10.1021/jacs.6b08808 |
| [34] |
J. Jiang, G.H. Ouyang, L. Zhang, M.H. Liu, Chem. -Eur. J. 23 (2017) 9439-9450. DOI:10.1002/chem.201700727 |
| [35] |
A. Rich, D.M. Blow, Nature (1958) 423. |
| [36] |
Y. Qiao, Y. Lin, Y. Wang, et al., Nano Lett. 9 (2009) 4500-4504. DOI:10.1021/nl9028335 |
| [37] |
Y. Gao, J. Hao, J. Wu, et al., Nanoscale 7 (2015) 13568-13575. DOI:10.1039/C5NR03699B |
| [38] |
J. Li, H. Cao, P. Liu, G. Cheng, M. Sun, Biomed Res. Int. (2014) 872139. |
| [39] |
A. Saha, J. Adamcik, S. Bolisetty, S. Handschin, R. Mezzenga, Angew. Chem. Int. Ed. 54 (2015) 5408-5412. DOI:10.1002/anie.201411875 |
 2019, Vol. 30
2019, Vol. 30