b Department of Chemistry, Beijing Normal University, Beijing 100875, China
Recently, the problem of skin hyperpigmentation has captured a great deal of consumers' attention. Actually, many products such as prescription, over-the-counter (OTC) and cosmetics, are on the market for the treatment of this kind of skin problem [1]. Unfortunately, it is not easy to find a topical regimen that fits with the requirements of safety and efficacy. Meanwhile, microbes play an important function in our lives, while some pathogenic bacteria are responsible for many infectious diseases, food safety, and ecological pollution [2]. Antibacterial agent is an important part of cosmetic, and is also the main means of preventing microbial contamination [3]. Since, most cosmetics are exposed to the skin for many times. Improper use of preservatives may cause the symptoms such as skin allergies and dermatitis [4-6]. Therefore, higher requirements are placed on the selection and use of cosmetic ingredients. Researchers are attempting to develop safe and effective preservatives and whitening agents.
Kojic acid (KA) is a metabolic product of various Aspergillus and Penicillium moulds, and has been extensively used as a food additive for preventing enzymatic browning and a cosmetic agent, for skin whitening [7]. It is the most popular agent employed for the treatment of melasma and preservation of food [8-10]. Furthermore, it can be combined with other skin lighteners and preservative. Unfortunately, it is very sensitive to sunlight, pH value and temperature [11]. KA is brown in both solution and cosmetic formulations because of its oxidation or reddening due to the complexation with metal ions such as irons [12]. Therefore, an effective solution is to choose an appropriate inorganic material as the host matrix to overcome such problems. These systems cannot only avoid direct contact with the skin, but also improve the thermal and optical stability of KA. More importantly, such systems utilize carriers that slowly release their contents in order to maintain antimicrobial and whitening concentrations at the desired levels for a longer period of time.
Layered double hydroxides (LDHs), whose formula can be generally written as [M2+1-xM3+x(OH) 2]x+(An-)x/n·mH2O (M2+ and M3+ are divalent and trivalent metal cations respectively, and An- is an exchangeable inorganic or organic anion), are 2D layered materials consisting of positively charged host layers with charge balancing guest anions [13]. One of the most interesting features of these materials is their role as a host matrix for the orientation and dispersion of interlayer anions. Therefore, LDHs show a very broad application in cosmetics, medicine, adsorbents, photochemistry and filler material [14-18]. Therefore, it can be anticipated that the incorporation of the complex functional organic acid into an LDH matrix will give rise to one kind of fine bacteriostatic and whitening agents.
In this paper, we describe the intercalation of KA into ZnTi-LDH. The details of the experiment are described in Supporting information. To avoid a direct contact with skin and optimize the cosmetic effect, ZnTi-LDH was chosen as lamellar host with the following advantages. Firstly, Zn and Ti are metals largely used both in pharmaceutical and cosmetic formulations, and the UV blocking ability of ZnTi-LDH almost ten times stronger than that of MgAl-LDH and ZnAl-LDH due to the presence of titanium element in the layers [19-22]. Secondly, the presence of the inorganic LDH host matrix may improve the thermal and optical stability of the intercalated KA. Finally, the organic-inorganic hybrid composite would have multi-function such as skin whitening, antibacterial and UV shielding properties. The supramolecular layered host-guest structure of ZnTi-KA-LDH was characterized by XRD, FT-IR, TG-DTA, and SEM. The inhibition of L-dopa oxidation, slow release and antibacterial activity of ZnTi-KA-LDH and KA have been studied and confirmed. The studies suggest that ZnTi-KA-LDH composites have potential applications as safe whitening and antimicrobial materials.
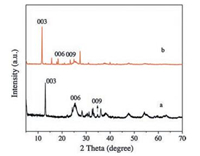
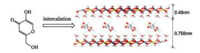
The ZnTi-LDH precursor and ZnTi-KA-LDH were synthesized respectively by co-precipitation and anion-exchange. The XRD patterns of ZnTi-LDH precursor and ZnTi-KA-LDH are shown in Fig. 1. The XRD pattern of the ZnTi-LDH precursor (Fig. 1a) exhibits typical characteristics of the LDH phase. The strong (003) peak reflection at 13.07° and the reflections of (006), (009) at 25.46°, 36.13°, which correspond to the basal and higher-order reflections [23]. The measured interlayer distance of ZnTi-LDH (d003 in Fig. 1a) is 0.677 nm, which is consistent with that in the literature report [24]. In the case of ZnTi-KA-LDH (Fig. 1b) the main characteristic reflections of the product appear at 11.66° (003), 18.33° (006), and 23.41° (009). The basal spacing for ZnTi-KA-LDH is 0.756 nm. The results indicated that the KA anions were successfully intercalated into the interlayer galleries of ZnTi-LDH. The structure model of ZnAl-KA-LDH is shown in Fig. 2.
|
Download:
|
| Fig. 1. XRD patterns of (a) ZnTi-LDH precursor and (b) ZnTi-KA-LDH. | |
|
Download:
|
| Fig. 2. Chemical structural formula of KA and the structure model of ZnTi-KA-LDH. | |
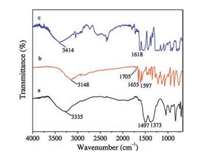
The FT-IR spectra of ZnTi-LDH precursor, pure KA and ZnTi-KA-LDH are shown in Fig. 3. The spectrum of ZnTi-LDH precursor (Fig. 3a) shows a strong broad absorption band between 3500 and 3000 cm-1 due to the O—H stretching mode of layer hydroxyl groups and of interlayer water molecules. The band at 1497 cm-1 together with its accompanying band at 1373 cm-1 is attributed to mode υ3 of interlayer carbonate species [25]. For pure KA (Fig. 3b), the typical bands are observed at 3148 cm-1 for O—H stretching vibration and around 1600 cm-1 due to the stretching vibration of carbonyl and C=C. The broad peak centered at about 3414 cm-1 is characteristic of all LDHs and arises from the νOH absorptions of the co-intercalated water molecules. The spectrum of the ZnTi-KA-LDH (Fig. 3c) showed a broad band between 3100 and 3600 cm-1, due to the stretches of hydrogen bonded hydroxyl group of both hydroxide layers and interlayer water. The characteristic peak representing carbonate ions disappeared. The results indicate that the KA anions have been successful intercalated into the interlayer galleries of the LDH. While the characteristic peak of the carbonate anion was absent, confirming that the carbonate groups have been completely displaced by the KA anions.
|
Download:
|
| Fig. 3. FT-IR spectra of (a) ZnTi -LDH precursor (b) KA and (c) ZnTi-KA-LDH. | |
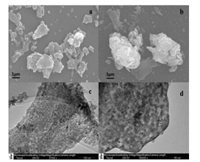
The SEM and TEM micrographs of the ZnTi-LDH precursor and ZnTi-KA-LDH are depicted in Fig. 4. The synthesized ZnTi-LDH (Fig. 4a) shows the presence of hierarchical structure consisting of two-dimensional thin nanoflakes, but some places are aggregated. From the SEM micrograph (Fig. 4b), it can be seen that the ZnTi-KA-LDH particles are aggregated and clearly exhibited the presence of hierarchical structure with the sizes range between 3 μm and 8 μm, aggregation result is related to the interaction among the flake-like crystals. The TEM micrographs of ZnTi-LDH (Fig. 4c) and ZnTi-KA-LDH (Fig. 4d) indicate the single-crystal-line nature of the ZnTi-LDH material.
|
Download:
|
| Fig. 4. SEM images of (a) ZnTi- LDH and (b) ZnTi-KA-LDH; TEM images of (c) ZnTiLDH and (d) ZnTi-KA-LDH. | |
Release behaviors of the ZnTi-KA-LDH and pure KA have been studied at pH 5 phosphate buffered solution (PBS). The release study was performed under this pH because the intact skin is acidic.
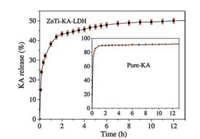
As shown in Fig. 5 (inset), pure KA has high initial KA release rate. The release profile of pure KA shows a high initial KA release amount in the initial 0.5 h and then reaches an almost constant level after 1 h. The release profiles are characteristic of a diffusioncontrolled release process [26]. On the other hand, ZnTi-KA-LDH (Fig. 5) shows a gradual release of intercalated kojic acid ions into the medium over a considerable time period. Even after 12 h, ZnTi-KA-LDH was still releasing the kojic acid anions.
|
Download:
|
| Fig. 5. Release profile of KA from ZnTi-KA-LDH in PBS (pH 5) and pure-KA (inset). | |
To study the release kinetic of anionic intercalated layered hydroxides hybrids, we picked out four comparably consistent kinetic models. The models are zeroth order (ⅰ) first order (ⅱ) second order (ⅲ) and Higuchi model (ⅳ):
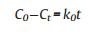
|
(1) |
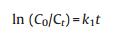
|
(2) |

|
(3) |
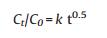
|
(4) |
C0 is the initial amount of guest KA in the solution, Ct is the amount of guest KA in the ZnTi-KA-LDH at time t, k is the rate constant. The fitting results are shown in Fig. 6, while a summary of the parameters extracted from the modeling can be found in Table 2. Usually, the larger correlation coefficients (R2) of the fitting equation is, the closer the fitting curve is to the release model. We can see from Table 1, comparing the R2 values of the four mathematical models, it was found that the R2 values were 0.964, 0.979, 0.956 and 0.995, respectively. The release data of ZnTi-KA-LDH is relatively fitted with the Higuchi model, which suggests that the release process is dominated by the dissolution of LDH particles and the ions exchange between the KA in the LDH and the ions present in the solution [27]. The result is consistent with the prolonged release behavior of kojic acid observed in Fig. 5b and its potential antimicrobial activity and whitening effect as observed in the following study.

|
Download:
|
| Fig. 6. Linear fitting of four models to the release of KA from (a) pure KA, (b) ZnTi-KA-LDH, according to zeroth order (ⅰ) first order (ⅱ) second order (ⅲ) and Higuchi model (ⅳ). | |
|
|
Table 1 Parameters extracted from the four fitting of the kinetic models. |
|
|
Table 2 The zones of pure KA and ZnTi-KA-LDH on three tested strains. |
KA anions from intercalated LDH materials did not completely release, similar to the other anions from pillared LDH [28]. This reason could be attributed to the possibility that kojic acid was deeply intercalated in the ZnTi-LDH host and the complete release would be very slow. Another reason may be the pH value of medium (H+ ions concentration), which leads to a proton attack to the kojic ions and thereby kojic ions get protonated and released to the medium. Therefore, the rate ofKAdiffusion outof the matrix is controlled by the effect of the layers matrix of LDH and the medium [29].
In the melanin synthesis pathway, tyrosinase can affect the conversion of tyrosine to dopa acid, and dopa acid oxidation to dopaquinone. Kojic acid, as a whitening agent, can inhibit melanin production by inhibiting tyrosinase activity. Therefore, its whitening efficacy can be evaluated by measuring the inhibitory effect on tyrosinase. The biochemical method is mainly used to detect whether the whitening effect ingredient has an inhibitory effect on tyrosinase. The common methods include the inhibition of tyrosinase experiment method and inhibition of L-dopa oxidation method [30]. In this experiment, inhibition of L-dopa oxidation method was adopted and L-dopa was used as the substrate. L-dopa, sample solution and phosphate buffer solution (pH 6.8) were added to separate tubes, the tubes were then placed in a constant temperature water bath at 37 ℃ for 5 min in a water bath. Then tyrosinase was used as positive control. The absorbance value was measured by UV–vis spectrophotometer at 475 nm and the inhibition rate was calculated [31-33].
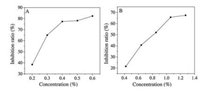
The dose-effect relationship of kojic acid in inhibiting the oxidation of L-dopa is shown in Fig. 7A. Kojic acid can inhibit L-dopa oxidation to a certain extent. In the range of 0.2%-0.4%, the oxidative inhibition rate of L-dopa increases with increasing concentration. When it was above 0.4%, the inhibition rate was less affected by the concentration, and the oxidative inhibition ability of L-dopa was gradually increased to the strongest. As shown in Fig. 7B, the abscissa is the concentration of kojic acid contained. Similar to the law of kojic acid inhibition rate, ZnTi-KA-LDH can effectively inhibit L-dopa oxidation. The results show that ZnTi-KA-LDH still has a whitening effect.
|
Download:
|
| Fig. 7. Inhibition rate curves for L-dopa of (A) KA and (B) ZnTi-KA-LDH. | |
In order to confirm the antimicrobial activities of pure KA and ZnTi-KA-LDH, we selected three test strains (Pseudomonas aeruginosa (ATCC15442), Escherichia coli (ATCC8739) and Staphylococcus aureus (ATCC6538)). The Young (24h old) type strains (50 μL, respectively) were coated on a solidified Nutrient Agar plates. The Oxford Cups were placed in Nutrient Agar plates. Each petri dish was placed three Oxford cups. Oxford cups were loaded with 150 μL of pure KA and ZnTi-KA-LDH by using a micropipette, respectively. The pure KA and ZnTi-KA-LDH were dissolved in PBS (pH 5). PBS (pH 5) was used as negative control. Finally, the plates were incubated at 37 ℃ in an incubator and observed after 16h. The antimicrobial activity of the pure KA and ZnTi-KA-LDH were tested in quadruplicate and the mean diameter of the zone inhibition zone was recorded.
As shown in Table 2, the pure KA and ZnTi-KA-LDH showed inhibitory activity against the E. coli, P. aeruginosa and S. aureus. No zone of inhibition was observed for the negative control— phosphate buffered solution (pH 5). The results showed that KA and ZnTi-KA-LDH had efficient inhibitory activity on the tested organisms, and pure KA and ZnTi-KA-LDH had better inhibitory effects against P. aeruginosa than the other two bacteria. Moreover, the pure KA showed a better antimicrobial activity on the three tested strains, when comparing with the pure KA and ZnTi-KA-LDH (in both, the concentration of KA is 100g/L). This may be due to incomplete dissolution of ZnTi-KA-LDH. This result was consistent with the slow release result. Furthermore, the enhanced antibacterial efficacy of ZnTi-KA-LDH is resulted by the synergistic effect of KA and ZnTi-LDH. The antibacterial efficacies of ZnTi-LDH and the other hydrotalcite with different lamellar elements, such as Mg, Ca, and Fe, are being studied and these results will be reported in our later paper.
Kojic acid, as a cosmetic agent, can inhibit the growth of microorganism as well as a preservative. E. coli, P. aeruginosa and S. aureus are normal flora of the human body; however, increased number of those bacteria can cause infection in some cases. The results gained from antibacterial testing showed that ZnTi-KA-LDH can inhibit the growth of microorganisms in acidic environment, which is similar with the human skin environment [34]. Combined with slow release experiment, the antibacterial activity resultsalso indicated that the antibacterial time of ZnTi-KA-LDH can be prolonged in acidic environment.
Anti-microbial behavior of pure KA and ZnTi-KA-LDH were studied at pH 5. Phosphate buffered solution (pH 5) was used as negative control.
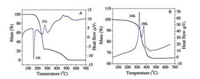
Fig. 8 shows TG-DTA profiles of pure KA and ZnTi-KA-LDH. The DTA curve of KA (Fig. 8A) shows an endothermic peak at 159 ℃ due to the melting point of the pure kojic acid. The peak after 200 ℃ may be due to the further decomposition and complete combustion of the residue. As shown in Fig. 8B, there are two exothermic peaks in the DTA curve of the ZnTi-KA-LDH, appearing at 346 ℃ and 396 ℃. The TG curve has two corresponding weight-loss stages. The first stage can be attributed to the decomposition of intercalated KA. It implies that intercalating KA in the LDH can improve the thermal stability of KA. The second stage was attributed to the combustion of residual organics.
|
Download:
|
| Fig. 8. TG-DTA curves of (A) KA and (B) ZnTi-KA-LDH. | |
In conclusion, XRD and FT-IR data indicated that KA anions have been successfullyintercalatedintoZnTi-LDH byan anion-exchange method. SEM and TEM images confirmed the typical hexagonal morphologyand the layering pattern of the resulting ZnTi-KA-LDH. TG data revealed that the thermal stability of KA is increased by intercalation into the galleries of LDH. The resulting ZnTi-LDH demonstrated slow and sustained release characteristics for kojic acid anions at pH 5, thus proving its applicability for antibacterial properties in cosmetics. The fitting of release data is best achieved with the Higuchi model, which suggests that the release of ZnTi-KA-LDH system is a heterogeneous diffusion process and release the guest in a slow and sustainable manner. Improved and prolonged activity of the novel nanohybrid has been proved the antimicrobial activity against the E. coli, P. aeruginosa and S. aureus, and the inhibition of L-dopa oxidation demonstrated that ZnTi-KA-LDH has a whitening effect. Therefore, ZnTi-KA-LDH has potential applications as antibacterial and whitening materials in cosmetic.
AcknowledgmentsThis work was supported by Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.03.050.
| [1] |
V. Ambrogi, L. Perioli, M. Nocchetti, et al., J. Phys.Chem.Solids 73 (2012) 94-98. DOI:10.1016/j.jpcs.2011.10.007 |
| [2] |
(a) Z. Wang, H. Yu, K. Ma, et al., Bioconjugate Chem. 29 (2018) 2090-2099; (b) Q. Yu, Z.Q. Wu, H. Chen, Acta Biomater. 16 (2015) 1-13. |
| [3] |
S.Y. Kwak, H.R. Choi, K.C. Park, Y.S. Lee, J. Pept. Sci. 17 (2011) 791-797. DOI:10.1002/psc.v17.12 |
| [4] |
V. Bugatti, G. Gorrasi, F. Montanari, Appl. Clay Sci. 52 (2011) 34-40. DOI:10.1016/j.clay.2011.01.025 |
| [5] |
V. Bugatti, U. Costantino, G. Gorrasi, et al., Eur. Polym. J. 46 (2010) 418-427. DOI:10.1016/j.eurpolymj.2009.11.003 |
| [6] |
A. Herman, O. Aerts, L. de Montjoye, et al., J. Eur. Acad. Dermatol. 33 (2019) 267-276. DOI:10.1111/jdv.2019.33.issue-2 |
| [7] |
S. Emami, S.J. Hosseinimehr, S.M. Taghdisi, et al., Med. Chem. Lett. 17 (2007) 45-48. DOI:10.1016/j.bmcl.2006.09.097 |
| [8] |
D.F. Li, P.P. Hu, M.S. Liu, et al., J. Agric. Food Chem. 61 (2013) 6597-6603. DOI:10.1021/jf401585f |
| [9] |
M. Vandeput, S. Patris, H. Silva, et al., Sens. Actuators B:Chem. 248 (2017) 385-394. DOI:10.1016/j.snb.2017.03.156 |
| [10] |
L. Lunadei, P. Galleguillos, B.D. Iglesias, Postharvest Biol. Technol. 60 (2011) 225-234. DOI:10.1016/j.postharvbio.2011.02.001 |
| [11] |
M. Gallarate, M.E. Parlotti, M. Trotta, A.E. Grande, C. Malarico, J. Cosmet. Sci. 55 (2004) 139-148. |
| [12] |
F. Azami, E. Tazikeh-Lemeski, M.A. Mahmood-Janlou, J. Chem. Health Risks. 7 (2017) 147-155. |
| [13] |
M. Fabiano de Almeida, C.R. Bellatoa, A.H. Mounteer, et al., Appl. Surf. Sci. 357 (2015) 1765-1775. DOI:10.1016/j.apsusc.2015.10.009 |
| [14] |
J. Li, Q.H. Fan, Y.J. Wu, J. Mater. Chem. A 4 (2016) 1737-1746. DOI:10.1039/C5TA09132B |
| [15] |
S. Barkhirdari, M. Yadollahi, Appl. Clay Sci. 121 (2016) 77-85. |
| [16] |
(a) R. Gao, D. Yan, et al., Chem. Sci. 8 (2017) 590-599; (b) Y.B. Dou, S.M. Xu, X.X. Liu, et al., Adv. Funct. Mater. 24 (2014) 514-521. |
| [17] |
(a) L. Moyo, S.S. Ray, W. Sebati, et al., J. Appl. Polym. Sci. 134 (2017) 27; (b) Y.B. Dou, T. Pan, S.M. Xu, et al., Angew. Chem. Int. Ed. 54 (2015) 9673-9678. |
| [18] |
(a) Y. Li, L. Tang, X. Ma, et al., J. Phys. Chem. Solids 107 (2017) 62-67; (b) A. Zhou, X.X. Liu, Y.B. Dou, et al., J. Mater. Chem. C 4 (2016) 8284-8290. |
| [19] |
X. Wang, Y. Li, L. Tang, et al., Chin. Chem. Lett. 28 (2017) 394-399. DOI:10.1016/j.cclet.2016.09.002 |
| [20] |
S. Lautenschlager, H.C. Wulf, M.R. Pittelkow, Photoprotection Lancet 370 (2007) 528-537. DOI:10.1016/S0140-6736(07)60638-2 |
| [21] |
W.Y. Shi, Y.J. Lin, S.T. Zhang, et al., Phys. Chem. Chem. Phys. 15 (2013) 18217-18222. DOI:10.1039/c3cp52819g |
| [22] |
G.R. Wang, S.M. Xu, C.H. Xia, et al., RSC Adv. 5 (2015) 23708-23714. DOI:10.1039/C5RA00589B |
| [23] |
Y.Z. Liu, Z.H. Yang, RSC Adv. 6 (2016) 68584-68591. DOI:10.1039/C6RA09096F |
| [24] |
O. Saber, H. Tagaya, J. Incl. Phenom. Macrocycl. Chem. 45 (2003) 107-115. DOI:10.1023/A:1023078728942 |
| [25] |
J.T. Kloprogge, D. Wharton, L. Hickey, R.L. Frost, Am. Mineral. 87 (2002) 623-629. DOI:10.2138/am-2002-5-604 |
| [26] |
P. Gomes, S. Hewageegana, J. Kottahachchi, et al., Sri Lankan J. Infect. Dis. 3 (2012) 32-39. |
| [27] |
M. Yang, L. Gu, B. Yang, et al., Appl. Surf. Sci. 426 (2017) 185-193. DOI:10.1016/j.apsusc.2017.07.207 |
| [28] |
P. Songkhum, T. Wuttikhun, N. Chanlek, P. Khemthong, K. Laohhasurayotin, Appl. Clay Sci. 152 (2018) 311-322. DOI:10.1016/j.clay.2017.11.028 |
| [29] |
A. Latip, M. Hussein, J. Stanslas, C. Wong, R. Adnan, Chem. Cent. J. 7 (2013) 119-129. DOI:10.1186/1752-153X-7-119 |
| [30] |
V.H. Sima, S. Patris, Z. Aydogmus, Talanta 83 (2011) 980-987. DOI:10.1016/j.talanta.2010.11.005 |
| [31] |
K. Tomita, N. Oda, M. Ohbayashi, et al., J. Antibiotics 43 (1990) 1601-1605. DOI:10.7164/antibiotics.43.1601 |
| [32] |
K.H. Kong, S.Y. Park, M.P. Hong, et al., Comp. Biochem. Physiol. Part B:Biochem. Mol. Biol. 125 (2000) 563-569. DOI:10.1016/S0305-0491(00)00163-2 |
| [33] |
S.S. Choi, H.S. Noh, S.H. Cho, et al., J. Pharm. Soc. Korea 45 (2001) 522-528. |
| [34] |
H. Lambers, S. Piessens, A. Bloem, et al., Int. J. Cosmet. Sci. 28 (2006) 359-370. DOI:10.1111/ics.2006.28.issue-5 |
 2019, Vol. 30
2019, Vol. 30