b Tianjin Key Laboratory for Photoelectric Materials and Devices, School of Materials Science and Engineering, Tianjin University of Technology, Tianjin 300384, China
With the development of portable electronics, such as curved smart phones, roll-up display panels, and wearable devices, an increasing demand in flexible energy storage devices has been aroused [1-9]. Among various energy storage devices, supercapacitors have attracted considerable attentions due to their high power density, superior rate capability, and excellent longterm cycling stability [10,11]. Therefore, flexible supercapacitors are extremely desired. The successful fabrication of flexible supercapacitors mainly depends on the preparation of high flexible and conductive electrodes as well as the design of novel device configurations [12]. Owing to the unique structures, nanocarbon, such as graphene and carbon nanotubes are often assembled into macroscopic architectures with large specific surface area and excellent mechanical properties to serve as the electrodes of flexible supercapacitors [13-20]. However, their preparation process is often complicated and cannot be scaled up in some cases. In addition to the above assembly, constructing nanocarbon or/and pseudocapacitive materials on the flexible conductive substrates (e.g., carbon cloth and carbon felts) is also a common method for fabricating the flexible electrodes of supercapacitors [21-25]. Such electrodes usually exhibit remarkable electrochemical and mechanical performance due to the synergistic effects of functional nanostructured materials and high-strength substrates. However, the electrochemically active materials are easily detached from the conductive substrates after repeated bending cycles, which would severely degrade the electrochemical performance. If nanostructured materials could be generated on the conductive substrates directly, above issues could be avoided and the electrochemical stability of electrodes would be improved.
Thermal treatment of carbon materials in air is a facile way to enhance their capacitive performance by increasing the specific surface area and oxygen-containing functional groups. With this method, Lu et al. achieved the surface engineering of carbon fiber paper, which shows a high areal capacitance of 0.75 F/cm2 [26]. Li et al. fabricated the activated wrinkled carbon membrane electrodes that exhibit a maximum specific capacitance of 154 F/g [27]. In addition, exfoliating nanocarbon from the surface of carbon materials could also endow them with the ability to remain the intrinsic electronic conductivity and increase electrochemical active sites [28], which is beneficial for improving their electrochemical performance. For instance, through electrochemical oxidation strategy, Lu et al. realized the exfoliation of carbon fibers with an improved areal capacitance of 0.76 F/cm2 [29]. Nevertheless, the electrochemical oxidation process is harmful to the environment due to the utilization of concentrated sulfuric acid and nitric acid. Thus, it is desired to develop efficient and ecofriendly strategies, which can not only allow for the formation of abundant oxygen-containing functional groups, but also exfoliate nanocarbon from the carbon fibers.
In this work, we report a thermal treatment strategy to fabricate graphene nanosheets, abundant micropores and oxygen-containing functional groups on the surface of ACFs. Commercial carbon felts (CFs) were chose as the raw material rather than carbon cloth or carbon paper due to the fact that CFs not only possess the common features of carbon fiber fabrics such as excellent conductivity, high chemical stability and good mechanical properties, but also have its unique advantages like higher porosity and more electrolyte uptake. The exfoliated graphene nanosheets endow ACFs with good electron conductivity and increased specific surface area, while the abundant micropores and oxygencontaining functional groups offer more active sites for the capacitance improvement, leading to the boost of electrochemical performance. As a result, the obtained ACFs electrodes exhibit superior areal capacitance, excellent rate ability, and cycling stability. Moreover, the assembled flexible supercapacitors based on ACFs electrodes also remain stable electrochemical performance even at various bending states.
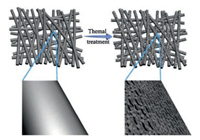
Fig. 1 schematically shows the thermally treating process of the ACFs. Typically, a piece of CFs was thermally treated in air at 400 ℃ for different time to obtain the ACFs. The prepared ACFs with different thermally treating time are named as ACFs-x, respectively, where x represents the thermal treatment time (x = 1, 2, 3, 4, 5, 6 and 7 h). After that, abundant oxygen-containing functional groups will be introduced onto the surface of CFs. Moreover, a small amount of surface C atoms can be transformed into carbon monoxide or carbon dioxide, leading to the formation of micropores and exfoliated graphene nanosheets. The detailed activation reactions can be understood as follows:
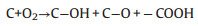
|
(1) |
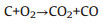
|
(2) |
|
Download:
|
| Fig. 1. Thermally treating process in preparing ACFs with exfoliated graphene nanosheets. | |
The scanning electron microscopy (SEM) images show that the raw CFs possess an interlaced network structure (Fig. S1a in Supporting information), which enables it with high electrolyte absorbability. It is noted that the carbon fibers in the raw CFs demonstrate a diameter of approximately 4–5 mm with smooth surface (Figs. 2a–c). After thermal treatment in air, graphene nanosheets and small pores are observed on the surface of carbon fibers. It is also reflected by their high-resolution transmission electron microscopy images (HRTEM, Figs. 2c and f). When the treatment time is 4 h, the amount of graphene nanosheets and small pores is higher than the cases of other treatment time. Furthermore, it is noted that the graphene nanosheets will disappear gradually after 4 h thermal treatment (Fig. S2 in Supporting information). Therefore, optimal thermal treatment time would be 4 h.

|
Download:
|
| Fig. 2. (a) Low-magnification and (b) High-magnification SEM images of the raw CFs (d) Low-magnification and (e) High-magnification SEM images of ACFs-4. (c) High-magnification TEM image of the raw CFs. (f) High-magnification TEM image of ACFs-4. | |
The X-ray photoelectron spectroscopy (XPS) measurements were performed to understand the chemical transformation from CFs to ACFs (Fig. 3a and Table S1 in Supporting information). It is noted that the intensity of O 1s peak is enhanced with the increase of thermal treatment since more oxygen-containing functional groups gradually form on the surface of CFs during the thermal treatment process. Furthermore, to understand the bonding configuration of the samples, high resolution XPS spectra of C 1s were carried out Figs. 3b and c). Compared with the case of CFs, the C-O (286.4 eV) and O-C=O (288.2 eV) peaks in the C 1s spectrum of ACFs-4 display higher intensities (Table S2 in Supporting information), also revealing that ACFs-4 possesses more surface oxygen-containing functional groups. It is also suggested by the O 1s spectra of ACFs-4 and raw CFs (Fig. S3 and Table S3 in Supporting information). In addition, it is noted that N 1s (399.7 eV) peaks was observed in the XPS spectra. The existence of nitrogen is also beneficial to the electrochemical performance due to the improved electronic properties and interfacial interaction of ACFs [18]. However, the content of nitrogen is nearly unchanged with the increase of thermally treating time.

|
Download:
|
| Fig. 3. (a) XPS spectra of the raw CFs and ACFs with different thermal treatment time. High resolution C 1s XPS spectra of (b) the raw CFs and (c) ACFs-4. (d) Raman spectra of the raw CFs and ACFs with different thermal treatment time, λ = 532 nm. (e) N2 absorption/desorption isotherm of ACFs with different thermal treatment time. (f) The calculated value of ID/IG and specific surface area of the raw CFs and ACFs with different thermal treatment time. | |
Apart from XPS results, Raman spectroscopy was also conducted to provide further insight into the chemical conversion from CFs to ACFs (Fig. 3d). Two feature peaks at 1353 and 1583 cm-1, corresponding to the D band and G band, respectively, are observed. The intensity ratio of D band to G band (ID/IG) is usually used for characterizing the defect concentration in graphite materials. In our case, the ID/IG of CFs is gradually rising from 1.00 to 1.11 with the continuous thermal treatment (Fig. 3f), indicating the increased surface defect concentration on ACFs induced by the introduction of oxygen-containing functional groups. The existence of oxygen-containing functional groups will improve the wettability of ACFs and then the ion diffusion kinetics could be further enhanced. It is proved by their contact angle measurements for water or 1.0 mol/L H2SO4 electrolyte (Fig. S4 in Supporting information). The corresponding contact angle of the raw CFs is 132.4° for water and 135.2° for 1.0 mol/L H2SO4 electrolyte, respectively, indicating the hydrophobic property of the raw CFs. However, the water and electrolyte droplets are completely absorbed immediately once they come into contact with the surface of ACFs. In addition, these species are electrochemical redox active sites and can introduce considerable pseudocapacitance to ACFs, which is beneficial to their electrochemical performance. Nevertheless, the introduction of too much oxygen-containing groups leads to the increased sheet resistance, which has impact on the electrochemical performance of ACFs (Fig. S5 in Supporting information). As a result, appropriate thermally treating time should be chosen based on the electrochemical performance of the ACFs.
The introduction of graphene nanosheets and small pores will lead to the increase of specific surface area, which is also required to meet the demand of high energy density. We further performed N2 adsorption-desorption isotherm (Fig. 3e) to evaluate the specific surface area of CFs with different thermal treatment time. Significantly, a type-I isotherm is observed in the ACFs (Fig. 3f), indicating the micropore structure of ACFs. Large specific surface areas ranging from 124.7 m2/g to 332.5 m2/g are achieved for the ACFs. Therefore, such ACFs demonstrate three distinct advantages in comparison with the traditional carbon fibers: 1) improved electrochemical activity, 2) good wettability of electrolyte and 3) high specific surface area, ensuring that they would show great potential in serving as high performance electrodes of supercapacitors.
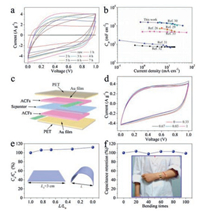
Since the ACFs are freestanding and conductive, they can be cut into slices and used as the electrodes of supercapacitors directly without the need for additional binder or conductive agents. To understand the electrochemical properties of the ACFs electrodes, symmetrical supercapacitors based on ACFs electrodes (mass per unit area of ACFs-4 is 8.4 mg/cm2) were assembled with 1.0 mol/L H2SO4 electrolyte. Cyclic voltammetry (CV) curves of the ACFs with different thermally treating time at the scan rate of 100 mV/s are shown in Fig. 4a and Fig. S6 (Supporting information). Typical rectangle like CV curves were obtained for the ACFs thermally treated at 400 ℃ with treating time less than 4 h and the ACFs-4 exhibits the higher specific areal capacitance (Fig. 4a and Fig. S7 in Supporting information). The enhancement of areal specific capacitance (when the activated time ≤ 4 h) is ascribed to the fact that prolonged thermally treating time results in the formation of lots of graphene nanosheets, increased specific surface area, and more oxygen-containing functional groups on the surface of carbon fiber. However, when the thermally treating time is more than 4 h, graphene nanosheets gradually disappear and the sheet resistance of the ACFs is also increased (Fig. S5 in Supporting information). As a result, the specific capacitances of ACFs thermally treated exceeding 4 h degrade fast. As a result, 4 h would be the optimal thermal treatment time. The cyclic voltammetry (CV) curves of ACFs-4 show a typical rectangle like shape at the scan rates ranging from 2 mV/s to 100 mV/s (Fig. S8 in Supporting information), implying the excellent double layer capacitive behavior. This is also proved by their galvanostatic charge/discharge (GCD) results (Fig. S9 in Supporting information). When the current density is 0.1 A/g, the gravimetric specific capacitance of ACFs-4 is about 188 F/g, corresponding to areal specific capacitance of 1579 mF/cm2 (for a single electrode). When the current density rises to 5.0 A/g, the ACFs-4 still operates well with a gravimetric specific capacitance of 122 F/g (areal specific capacitance: 1025 mF/cm2). The large areal capacitances and excellent rate performance of ACFs-4 are comparable to those of recently reported carbon fiber based materials, such as carbon paper and carbon cloth (Fig. 4b) [26,29-33]. In addition, the ACFs-4 possesses a prominent cycling stability with retention of more than 99% of its initial capacitance after 1000 cycles at the current density of 2.0 A/g (Fig. S10 in Supporting information).
|
Download:
|
| Fig. 4. (a) CV curves of supercapacitors based on the raw CFs and ACFs (scan rate: 100 mV/s). (b) Comparison of areal specific capacitances as a function of the current densities between ACFs-4 and previously reported electrodes. (c) Schematic diagram of the flexible all-solid-state supercapacitor. (d) CV curves at the scan rate of 2 mV/s under different values of L/L0, where L0 is the initial length of the device; L is the distance between two ends of the device at varied bending levels. (e) The normalized areal specific capacitance at varied bending states, where Ca0 and Ca are the initial areal capacitance and the areal specific capacitance at varied bending levels. (f) Capacitance retention of the flexible all-solid-state supercapacitor device at different bending times. The capacitance at original state is normalized to 1 (Inset is the optical image of an LED illuminated by two devices in series on an arm). | |
Owing to the excellent mechanical properties, ACFs are able to be bent even at large bending states. As a result, it can act as the electrodes of flexible supercapacitors (Fig. S11a in Supporting information). Based on ACFs-4, a flexible all-solid-state symmetric supercapacitor device was fabricated by sandwiching the separator and PVA/H2SO4 polymer gel electrolyte between the ACFs-4 electrodes, as depicted in Fig. 4c. The CV curves (Fig. 4d) of the device exhibit very small distinction when the distance between two ends of the device changes from 3 cm to about 0 under bending states (Figs. S1b–f in Supporting information). The normalized specific capacitances of the device at varied bending states were also calculated (Fig. 4e) (The capacitance under flat state (L/L0 = 1) is 1215 mF/cm2). A slight increase in the specific capacitances can be seen clearly with the bending degree enhanced, which is ascribed to the better contact between carbon fibers and Au film current collectors. Furthermore, there is only a little change in the capacitance retention of the flexible all-solid-state supercapacitor device even after repeated bending 100 times or 1000 cycles (Fig. 4f and Fig. S12 in Supporting information). These indicate that the all-solid-state supercapacitor device based on ACFs-4 can serve as flexible energy storage devices. To illustrate the flexibility of the supercapacitor device by a simple visual cue, two devices were connected in series and fixed on an arm to light a red LED under bending state inset in Fig. 4f). The resultant devices were powerful enough to light up a red LED, which reveals the excellent applicability and compatibility of portable electronics.
In summary, ACFs were prepared by a simple thermal treatment strategy. The uniformly distributed graphene nanosheets, abundant micropores, and appropriate amount of oxygen-containing functional groups were successfully introduced on the surface of ACFs, which lead to a significant improvement of the specific surface area and electrochemical activity. Therefore, ACFs show excellent electrochemical performance compared with the commercial CFs. Furthermore, based on ACFs, flexible all-solid-state supercapacitors can be assembled and they can still remain stable electrochemical performance under different bending states. As a result, this strategy could broaden the applications of commercial CFs in portable, flexible, and wearable energy-storage devices.
AcknowledgmentsThis work was supported by National Natural Science Foundation of China (Nos. 21573116, 51822205 and 21875121), Ministry of Science and Technology of China (No. 2017YFA0206701), Ministry of Education of China (No. B12015), and the Young Thousand Talents Program.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.03.003.
| [1] |
X.J. Wu, Y.J. Xu, Y. Hu, et al., Nat. Commun. 9 (2018) 4573. DOI:10.1038/s41467-018-06914-7 |
| [2] |
X. Pu, L.X. Li, M.M. Liu, et al., Adv. Mater. 28 (2016) 98-105. DOI:10.1002/adma.201504403 |
| [3] |
Z.B. Yang, J. Deng, X.L. Chen, J. Ren, H.S. Peng, Angew. Chem. Int. Ed. 52 (2013) 13453-13457. DOI:10.1002/anie.201307619 |
| [4] |
J. Lv, I. Jeerapan, F. Tehrani, et al., Energy Environ. Sci. 11 (2018) 3431-3442. DOI:10.1039/C8EE02792G |
| [5] |
P.S. Luan, N. Zhang, W.Y. Zhou, et al., Adv. Funct. Mater. 26 (2016) 8178-8184. DOI:10.1002/adfm.201603480 |
| [6] |
Y. Khan, A.E. Ostfeld, C.M. Lochner, A. Pierre, A.C. Arias, Adv. Mater. 28 (2016) 4373-4395. DOI:10.1002/adma.v28.22 |
| [7] |
D.H. Kim, J. Viventi, J.J. Amsden, et al., Nat. Mater. 9 (2010) 511-517. DOI:10.1038/nmat2745 |
| [8] |
S. Huang, F. Wan, S.S. Bi, et al., Angew. Chem. Int. Ed. 58 (2019) 4313-4317. DOI:10.1002/anie.201814653 |
| [9] |
J.R. Tian, C.J. Cui, C. Zheng, W.Z. Qian, Chin. Chem. Lett. 29 (2018) 599-602. DOI:10.1016/j.cclet.2018.01.027 |
| [10] |
Z.Q. Niu, L. Zhang, L.L. Liu, et al., Adv. Mater. 25 (2013) 4035-4042. DOI:10.1002/adma.v25.29 |
| [11] |
M.M. Hu, J.Q. Wang, J. Liu, et al., Chem. Commun. 54 (2018) 6200-6203. DOI:10.1039/C8CC03375G |
| [12] |
L.Q. Yao, T. Cheng, X.Q. Shen, et al., Chin. Chem. Lett. 29 (2018) 587-591. DOI:10.1016/j.cclet.2018.01.007 |
| [13] |
Q.R. Wang, X.Y. Wang, F. Wan, et al., Small 14 (2018) 1800280. DOI:10.1002/smll.v14.23 |
| [14] |
Y.W. Zhu, S. Murali, M.D. Stoller, et al., Science 332 (2011) 1537-1541. DOI:10.1126/science.1200770 |
| [15] |
J. Chmiola, G. Yushin, Y. Gogotsi, et al., Science 313 (2006) 1760-1763. DOI:10.1126/science.1132195 |
| [16] |
C. Chen, J. Cao, Q.Q. Lu, et al., Adv. Funct. Mater. 27 (2017) 1604639. DOI:10.1002/adfm.v27.3 |
| [17] |
N. Rey-Raap, M. Enterria, J.I. Martins, M.F.R. Pereira, J.L. Figueiredo, ACS Appl. Mater. Interfaces 11 (2019) 6066-6077. DOI:10.1021/acsami.8b19246 |
| [18] |
Q.H. Geng, G.X. Huang, Y.B. Liu, et al., Electrochim. Acta 298 (2019) 1-13. DOI:10.1016/j.electacta.2018.12.038 |
| [19] |
L. Zhang, W.X. Liu, W.H. Shi, et al., Chem. Eur. J. 24 (2018) 13792-13799. DOI:10.1002/chem.201802826 |
| [20] |
X.L. Xu, W.H. Shi, P. Li, et al., Chem. Mat. 29 (2017) 6058-6065. DOI:10.1021/acs.chemmater.7b01947 |
| [21] |
S.Y. Wang, R.A.W. Dryfe, J. Mater. Chem. A Mater. Energy Sustain. 1 (2013) 5279-5283. DOI:10.1039/c3ta10436b |
| [22] |
Y. Yang, Q.Y. Huang, L.Y. Niu, et al., Adv. Mater. 29 (2017) 1606679. DOI:10.1002/adma.v29.19 |
| [23] |
L.B. Dong, C.J. Xu, Q. Yang, et al., J. Mater. Chem. A Mater. Energy Sustain. 3 (2015) 4729-4737. DOI:10.1039/C4TA06494A |
| [24] |
X.L. Xu, W.H. Shi, W.X. Liu, et al., J. Mater. Chem. A Mater. Energy Sustain. 6 (2018) 24086-24091. DOI:10.1039/C8TA06412A |
| [25] |
K. Zhu, Y. Wang, J.A. Tang, et al., Mater. Chem. Front. 1 (2017) 958-966. DOI:10.1039/C6QM00196C |
| [26] |
H.Z. Zhang, W.D. Qiu, Y.F. Zhang, et al., J. Mater. Chem. A Mater. Energy Sustain. 4 (2016) 18639-18645. DOI:10.1039/C6TA08138J |
| [27] |
X.X. Yu, Y. Wang, L. Li, H.B. Li, Y.Y. Shang, Sci. Rep. 8 (2018) 45378. |
| [28] |
Z.J. Liu, Z.H. Zhao, Y.Y. Wang, et al., Adv. Mater. 29 (2017) 1606207. DOI:10.1002/adma.201606207 |
| [29] |
W. Wang, W.Y. Liu, Y.X. Zeng, et al., Adv. Mater. 27 (2015) 3572-3578. DOI:10.1002/adma.v27.23 |
| [30] |
Z.F. Wang, Y. Han, Y.X. Zeng, et al., J. Mater. Chem. A Mater. Energy Sustain. 4 (2016) 5828-5833. DOI:10.1039/C6TA02056A |
| [31] |
P. Suktha, P. Chiochan, P. Iamprasertkun, et al., Electrochim. Acta 176 (2015) 504-513. DOI:10.1016/j.electacta.2015.07.044 |
| [32] |
G.M. Wang, H.Y. Wang, X.H. Lu, et al., Adv. Mater. 26 (2014) 2676-2682. DOI:10.1002/adma.201304756 |
| [33] |
S.L. Jiang, T.L. Shi, X.B. Zhan, et al., J. Power Sources 272 (2014) 16-23. DOI:10.1016/j.jpowsour.2014.08.049 |
 2019, Vol. 30
2019, Vol. 30 

