b Beijing National Laboratory for Molecular Science, Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China;
c College of Chemistry and Material Science, Shandong Agriculture University, Tai'an 271018, China;
d Tianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, School of Science, Tianjin University and Collaborative Innovation Center of Chemical Science and Engineering(Tianjin), Tianjin 300072, China
In the past decades, organic field-effect transistors (OFETs) has become one frontier and hot research topics in organic electronics because of its broad application from sensing to flexible circuits [1-7]. As the core part of the OFETs, organic semiconductors have also witnessed rapid development in terms of molecular varieties, structure modifications as well as packing arrangements [8-10]. Pentacene, the benchmark material of OFETs semiconductor, exhibits poor stability in ambient conditions and low solubility in organic solvents, hindering its widespread use in OFETs [11-13]. Therefore, design and synthesize new semiconductors with high stability, excellent properties and good solubility are in demand for the practical application of OFETs [14,15].
Thienoacenes, characterized by fused thiophene rings in the backbones of molecules, is the most popular group of semiconducting materials applied in OFETs in recent decade [16-20]. The infinite molecular structures and various intermolecular interactions make the packing arrangement compact and beneficial for charge transport. Physical properties comparison of thienoacenes and acenes with the same number of fused rings indicated that the replacement of phenylenes with thiophene make the highest occupied molecular orbit (HOMO) energy levels of thienoacenes are much lower than those of acenes. What are also improved are the greatly increased energy gaps (Eg) of thienoacenes, which make thienoacenes possess higher environmental stabilities than corresponding acenes. All these mentioned enhancements allow thienoacenes to become promising candidates of semiconducting materials for OFETs
Pentathienoacene (PTA) possesses similar rigidity, coplanar π-conjugated framework structure and the same number of delocalized π-electrons to pentacene, however, the low mobility, poor solubility and tedious synthetic route are the bottleneck for the development in OFETs [21]. Previous studies have shown that the introduction of alkyl chains on π-conjugated framework can not only improve solubility, but also enhance the intermolecular interaction, which are critical to increase π-orbital overlap among adjacent molecules [22-26]. Herein, a novel dihexyl-substituted pentathienoacene (C6-PTA) is designed and synthesized (Scheme 1). C6-PTA possesses high thermal stability (305 ℃), wide band gap (3.24 eV) and suitable HOMO levels (5.29 eV) as revealed by the thermogravimetric analysis (TGA), UV–vis absorption spectroscopy and cyclic voltammetry. Compared with PTA, the introduction of hexyl chains endow the thin film semiconductor with about threefold increase in carrier mobility and one to three orders of magnitude improvement in current on/ off ratio. Furthermore, single crystal FETs based on C6-PTA exhibited mobility up to 0.64 cm2 V-1 s-1, which is over 50 times higher than the thin film counterpart.
|
Download:
|
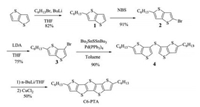
| Scheme 1. Synthetic route of C6-PTA. | |
The detailed synthesis of C6-PTA with the total yield of 25.2% is shown in Scheme 1. The introduction of hexyl group to thieno[3, 2-b]thiophene carried out by reacting with n-butyl lithium (n-BuLi) and 1-bromohexane. After bromination by NBS, the bromide 2-bromo-5-hexylthieno[3, 2-b]thiophene 2 converted to 6-bromo- 2-hexylthieno[3, 2-b]thiophene 3 by bromine dance reaction. The synthesis of sulfide 4 was achieved by the Pd-catalyzed Stille coupling of Bu3SnSSnBu3 with 2 equiv. of 3 in high yield up to 90%. There is no denying that the sulfide coupling method is a huge improvement compared to the tedious and inefficient Br-Li exchange reaction. Finally, the sulfide 4 was lithiated with n-BuLi in THF, and then reacted with copper chloride as a coupling reagent to give target compound with a yield of 50%.
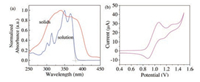
The UV–vis absorption spectrum of C6-PTA in CH2Cl2 solution is shown in Fig. 1a. The absorption peaks at λ = 365 and 348 nm can be assigned to the 0–0 and 0–1 transitions to the S1 state (π-π*). The peaks at 314 and 302 nm can be assumed to the absorption along short axis similar to the case of TIPS-pentacene [27]. We also measured the absorption spectrum in solid state using integrating sphere method after grinding the crystals into powers. A new shoulder peak appears at 382 nm, which can be attributed to closed stacking induced intermolecular excition absorption. The optical bandgap of C6-PTA in solution is determined to be 3.24 eV by extrapolating the long-wavelength absorption edge using the equation Eg = 1240/λonset eV. This value is close to that of PTA (3.2 eV), suggesting that the introduction of alkyl chains did not influence the band gap. In addition, band gap of PTA and C6-PTA are much larger than that of pentacene (1.85 eV), indicative of much higher stability than pentacene. The cyclic voltammetry of C6-PTA in CH2Cl2 shows one reversible oxidation wave using glass carbon electrode as a working electrode and Ag/AgCl as reference electrode (Fig. 1b). The HOMO level of C6-PTA calculated to be -5.29 eV, determined by the onset position of the oxidation peak according to the literature. The low-lying HOMO level confirms the high oxidation stability of C6-PTA. The thermal property of C6-PTA was investigated by thermogravimetirc analysis (TGA) and differential scanning calorimetry (DSC) as illustrated in Figs. S1 and S2 in Supporting information, respectively. TGA result indicates that compound C6-PTA has a good thermal stability and temperature of weight loss is at 305 ℃, which is 33 ℃ higher than that of unsubstituted PTA. This result signifies from another perspective that introduction of the alkyl chains improves the thermal stability of thienoacene derivatives. The DSC figure shows a phase transition at 167 ℃ and a melting point at 184 ℃, which indicate that the ambient crystal phase is stable under 167 ℃.
|
Download:
|
| Fig. 1. UV–vis spectrum (a) and cyclic voltammogram (b) of C6-PTA. | |
The OFET behaviours of C6-PTA were explored using the top contact device structure on unmodified SiO2/Si and OTS modified substrates. The results show that all the devices show typical p-type channel FET properties under ambient conditions. Figs. 2a and b show typical FET characteristics of C6-PTA deposited at room temperature. The I–V characteristics show standard linear and saturation regions. Interestingly, the FET performances did not display substrate temperature dependence. A high mobility up to 0.012 cm2 V-1 s-1 as well as on/off ratio over 106 are obtained on modified SiO2/Si substrates, which far outperformed the PTA based thin film transistors with a mobility as low as 0.0043 cm2 V-1 s-1 and on/off ratio in range of 103 to 105 [21]. Compared with the modified SiO2/Si substrates, the OFET performance of unmodified SiO2/Si substrates is much lower (0.009 cm2 V-1 s-1, Fig. S3 in Supporting information).

|
Download:
|
| Fig. 2. Transfer (a) and output (b) curves of C6-PTA film devices, XRD (c) and AFM (d) of C6-PTA films on OTS modified SiO2/Si substrates. | |
The microstructure of the thin film was characterized by X-ray diffraction (XRD) and atomic force microscope (AFM) (Figs. 2c and d). The XRD measurement of C6-PTA thin films deposited on octadecyl-trichlorosilane (OTS) modified and unmodified SiO2/Si substrates show similar diffraction peaks. There are four diffraction peaks at 4.738, 9.368, 18.748 and 33.648, corresponding to the firstorder, second-order, third-order and fourth-order diffraction. The primary peak shows strong diffraction and the d-spacing is estimated to be 2.06 nm, agreeing well with the length of the C6-PTA molecule. This result indicates that the molecules are stacked nearly perpendicular to the substrate. This edge-on orientation has found to be favourable to efficient charge transport since the stacking direction is consistent with the current flowing direction. Compared with the OTS modified SiO2/Si, the diffraction peaks of film on unmodified substrates is weaker and the baseline is not smooth, indicating low degree of order (Fig. S4 in Supporting information).
To further explore the morphology of C6-PTA in thin films and understand the effect on the substrate-dependent mobility, the surface morphology of thin films of C6-PTA was explored with AFM. The result show that the morphology of thin films strongly depends on the substrates. The average grain size is determined to be ~37 nm on modified SiO2/Si substrate while it decrease to ~28 nm on unmodified counterpart (Fig. S5 in Supporting information) [28]. This variation tendency of thin film morphology along with the depositing conditions is coincident with the OFET performance since the thin film with bigger grain size and less grain boundary is helpful for charge transport.
Due to the long-range order in nature, single crystal can act as a powerful tool to reveal the intrinsic properties of semiconductor. Therefore, devices based on the single crystal of C6-PTA were also fabricated. The transmission electron microscope (TEM) and selected area electron diffraction (SAED) (Fig. 3a) prove the formation of single crystals. The image of the single-crystal device was shown in Fig. 3b. Typical FET characteristics of single crystal devices are shown in Fig. 3c and d. From the saturation regime of the transfer characteristics, a mobility up to 0.64 cm2 V-1 s-1 as well as on/off ratio over 106 is calculated. Thus, we achieved nearly 50 times increase in carrier mobility for single crystals relative to the thin film counterpart.

|
Download:
|
| Fig. 3. SAED and TEM (inset figure) of single crystal (a), the device image (b), transfer (c) and output (d) curves of C6-PTA single crystal devices. | |
In summary, a novel hexyl substituted pentathienoacene C6-PTA is designed and synthesized. UV–vis spectra, electrochemistry and TGA results show that this material has a large HOMOLUMO bandgap, a low-lying HOMO level and good thermal stability. The introduction of hexyl chains allows about threefold increase in carrier mobility and one to three orders of magnitude improvement in current on/off ratio. Moreover, single crystal FETs based on C6-PTA exhibited mobility up to 0.64 cm2 V-1 s-1, which is over 50 times higher than the thin film counterpart. All results indicate that C6-PTA is a promising organic semiconductor with high mobility and stability.
AcknowledgmentsWe are grateful for the financial support from the Ministry of Science and Technology of China (Nos. 2015CB856502, 2016YFB0401100 and 2017YFA0204503), the National Natural Science Foundation of China (Nos. 51733004, 51822308, 51725304, 51633006, 21661132006), the Strategic Priority Research Program (No. XDB12000000), and the Youth Innovation Promotion Association of the Chinese Academy of Sciences, Startup Foundation for Doctors of China West Normal University (No. 15E007), Open Foundation of Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province (No. CSPC2015-1-1).
| [1] |
H. Li, W. Shi, J. Song, et al., Chem. Rev. 119 (2019) 3-35. DOI:10.1021/acs.chemrev.8b00016 |
| [2] |
X. Wu, S. Mao, J. Chen, et al., Adv. Mater. 30 (2018) 1705642. DOI:10.1002/adma.v30.17 |
| [3] |
H. Sirringhaus, Adv Mater. 26 (2014) 1319-1335. DOI:10.1002/adma.201304346 |
| [4] |
H.B. Li, G.B. Xue, J.K. Wu, et al., Chin. Chem. Lett. 28 (2017) 2121-2124. DOI:10.1016/j.cclet.2017.08.011 |
| [5] |
J. Xu, S. Wang, G.J.N. Wang, et al., Science 549 (2017) 59-64. |
| [6] |
L.N. Fu, B. Leng, Y.S. Li, et al., Chin. Chem. Lett. 29 (2018) 175-178. DOI:10.1016/j.cclet.2017.05.014 |
| [7] |
Y.Q. Liu, M. Santella, Z.Q. Fan, et al., Chin. Chem. Lett. 29 (2018) 271-275. DOI:10.1016/j.cclet.2017.08.034 |
| [8] |
C.L. Wang, H.L. Dong, W.P. Hu, Y.Q. Liu, D.B. Zhu, Chem. Rev. 112 (2012) 2208-2267. DOI:10.1021/cr100380z |
| [9] |
Y.G. Zhen, H.L. Dong, L. Jiang, et al., Chin. Chem. Lett. 27 (2016) 1330-1338. DOI:10.1016/j.cclet.2016.06.023 |
| [10] |
G. Bolla, H. Dong, Y. Zhen, et al., Sci. China Mater. 59 (2016) 523-530. DOI:10.1007/s40843-016-5049-y |
| [11] |
Y.Y. Lin, D.J. Gundlach, S.F. Nelson, T.N. Jackson, IEEE Electron. Device Lett. 18 (1997) 606-608. DOI:10.1109/55.644085 |
| [12] |
H. Okamoto, N. Kawasaki, Y. Kaji, et al., J. Am. Chem. Soc. 130 (2008) 10470-10471. DOI:10.1021/ja803291a |
| [13] |
C.D. Sheraw, T.N. Jackson, D.L. Eaton, J.E. Anthony, Adv. Mater. 15 (2003) 2009-2011. DOI:10.1002/adma.200305393 |
| [14] |
M. Watanabe, W.T. Su, K.Y. Chen, et al., Chem. Commun. 49 (2013) 2240-2242. DOI:10.1039/c3cc00278k |
| [15] |
M. Watanabe, Y.J. Chang, S.W. Liu, et al., Nat Chem. 4 (2012) 574-578. DOI:10.1038/nchem.1381 |
| [16] |
X.N. Zhang, A.P. Côté, A.J. Matzger, J. Am. Chem. Soc. 127 (2005) 10502-10503. DOI:10.1021/ja053326m |
| [17] |
J.H. Gao, R.J. Li, L.Q. Li, et al., Adv. Mater. 19 (2007) 3008-3011. DOI:10.1002/adma.200701167 |
| [18] |
H. Ebata, T. Izawa, E. Miyazaki, et al., J.Am. Chem.Soc. 129 (2007) 15732-15733. DOI:10.1021/ja074841i |
| [19] |
M.L. Tang, T. Okamoto, Z. Bao, J. Am. Chem. Soc. 128 (2006) 16002-16003. DOI:10.1021/ja066824j |
| [20] |
M.L. Tang, S.C.B. Mannsfeld, Y.S. Sun, et al., J. Am. Chem. Soc. 131 (2009) 882-883. DOI:10.1021/ja808142c |
| [21] |
K. Xiao, Y. Liu, T. Qi, et al., J. Am. Chem. Soc. 127 (2005) 13281-13286. DOI:10.1021/ja052816b |
| [22] |
H. Ebata, T. Izawa, E. Miyazaki, et al., J. Am. Chem. Soc. 129 (2007) 15732-15733. DOI:10.1021/ja074841i |
| [23] |
H. Minemawari, T. Yamada, H. Matsui, et al., Nature 475 (2011) 364-367. DOI:10.1038/nature10313 |
| [24] |
Y. Miyata, E. Yoshikawa, T. Minari, et al., J. Mater. Chem. 22 (2012) 7715-7717. DOI:10.1039/c2jm30840a |
| [25] |
Y. Diao, B.C. Tee, G. Giri, et al., Nat. Mater. 12 (2013) 665-671. DOI:10.1038/nmat3650 |
| [26] |
P. He, Z.Y. Tu, G.Y. Zhao, et al., Adv. Mater. 27 (2015) 825-830. DOI:10.1002/adma.v27.5 |
| [27] |
M.S. Niu, F. Zheng, X.Y. Yang, et al., Org. Electron. 49 (2017) 340-346. DOI:10.1016/j.orgel.2017.07.007 |
| [28] |
C.K. Lv, F. Zheng, X.Y. Yang, et al., J. Phys. Chem. C 122 (2018) 2572-2581. DOI:10.1021/acs.jpcc.7b11726 |
 2019, Vol. 30
2019, Vol. 30 

