b Department of Radiology, China-Japan Friend Hospital, Beijing 100029, China
Pancreatic cancer is a devastating disease that has few improvement in its mortality rate in the last decade, and is largely refractory to currently available treatment strategies [1, 2]. The 5-year survival of pancreatic cancer is only 3%–5%, and the incidence is increasing in many countries [3, 4]. Therefore, extensive researches were focused on treatment of pancreatic cancer. Chemotherapy becomes one of the traditional treatments for pancreatic cancer in recent decades [5]. However, a major obstacle of chemotherapy in pancreatic cancer treatment is the low curative rate, which limits the further clinical application of chemotherapy [6]. Moreover, huge therapeutic dose is required because of aimless distribution and rapid elimination in vivo, leading to severe side effects [7]. Therefore, chemotherapy for cancer needs to be improved for better therapeutic effects and lower toxicity.
Gambogic acid (GA) is the principal active component of gamboge resin, and it has extensive biological activity, such as antiinflammatory, anti-viral, anti-infectious, and anti-oxidant [8, 9]. In recent years, the anticancer effect of GA was found by pharmaceutical scientists [10, 11]. GA shows strong inhibitory effects on many kinds of tumor cells, including breast cancer, lung carcinoma, gastric cancer, hepatocarcinoma, prostate cancer, melanoma, and pancreatic cancer [12-18]. However, the poor water solubility, rapid blood clearance, and nonselective cytotoxicity of GA limit its clinical application [19-21]. So, strategy which overcomes above drawbacks is urgent to be developed.
In recent years, nanotechnology was developed rapidly and applied in drug delivery and cancer therapy [6, 22-26]. Novel drug delivery system (DDS) such as biodegradable polymeric micelles was carried out to meet these challenges of hydrophobic chemotherapeutic agents [23]. Hydrophobic drug was encapsulated into polymeric micelles, and showed preferable water dispersion, stability, and drug release behavior in vitro [27]. In addition, drug-loaded polymeric micelles which possess long blood circulation time in vivo due to the nano-size and the presence of the hydrophilic shell could passively target the tumor site by the enhanced permeability and retention (EPR) effect, therefore improving anti-tumor therapeutic outcomes [27-29].
In this study, monomethyl poly(ethylene glycol)-poly(ε-caprolactone)-poly(trimethylene carbonate) (MPEG-P(CL-ran-TMC)) copolymers were successfully synthesized by ring-opening polymerization. The non-crystallization TMC in copolymers could prevent the crystallization of the PCL to improve the micelles stability [30, 31]. GA encapsulated MPEG-P(CL-ran-TMC) micelles (GA micelles) were successfully prepared by a single-step solid dispersion method. Drug release behavior, cellular uptake, cytotoxicity, and apoptosis induction were studied in vitro. Furthermore, in vivo antitumor activity, apoptosis-inducing and anti-proliferation in tumors were measured. Our work provided a potential treatment approach for pancreatic cancer by GA encapsulated MPEG-P(CL-ran-TMC) micelles.
First of all, MPEG-P(CL-ran-TMC) copolymer was successfully prepared by ring opening polymerization of ε-CL and TMC initiated by MPEG and stannous octoate was used as catalyst. MPEG-P(CL-ran-TMC) was successfully synthesized (Fig. S1 in Supporting information) and characterized by 1H NMR. The molecular weight of the synthesized MPEG-P(CL-ran-TMC) copolymer was 4431 (MPEG/PCL/PTMC ratio: 1995/1824/612) calculated by 1H NMR.
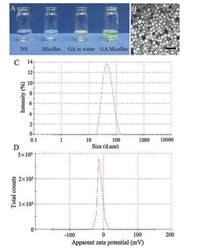
After the material was successfully synthesized, GA micelles were prepared by a single-step solid dispersion method (Table S1 in Supporting information, sample S3 was used in our work). As shown in Fig. 1A, GA micelles in water formed a clear and transparent solution, while free GA formed a turbid suspension in water because of its poor hydrophilicity. It indicated that nanoformulation of GA could significantly improve water dispersion of GA. The morphological characteristics of GA micelles were detected by TEM, in which GA micelles showed a well-defined spherical structure (Fig. 1B). The particle size and zeta potential of GA micelles were detected by a Malvern laser particle size analyzer. Fig. 1C demonstrated that the average particle size of GA micelles was 44 ±1 nm with a polydisperse index (PDI) of 0.146 ± 0.012. Fig. 1D showed that the zeta potential of GA micelles was -11.55 ±1.25 mV. The drug loading (DL) and encapsulation efficiency (EE) of GA micelles was detected by HPLC, and the DL of GA micelles was 26.28% ± 0.12% with EE of 87.59% ± 0.41%. In short, GA micelles with high DL and EE were prepared by a onestep solid dispersion method, which showed good water dispersion and uniform spherical structure.
|
Download:
|
| Fig. 1. Characterization of GA micelles. (A) Appearance of water, blank micelles, GA in water, and GA micelles. (B) TEM image of GA micelles, Scale bar = 100 nm. (C) Size distribution of GA micelles. (D) Zeta potential of GA micelles. | |
Next, the property of GA micelles as a drug delivery system was investigated. A dialysis method was performed to evaluate the drug release behaviors of free GA and GA micelles in vitro. The release media was collected, and the content of GA in release media was detected by HPLC. The result was showed in Fig. 2D, 50.51% ± 1.19% of free GA was released in the external media at 24 h, while only 19.85% ± 1.38% of GA micelles was released at the same time. At 240 h, free GA has released up to 88.16% ± 2.78%, while 55.03% ± 0.64% of GA micelles has released in the external media. The controlled release behavior of GA micelles indicated their potential applicability as a drug delivery system to prolong retention time in blood vessels while minimizing exposure to healthy tissues.

|
Download:
|
| Fig. 2. Cytotoxicity studies and drug release study of GA micelles. Cell viability of AsPC-1 cells treated with free GA or GA micelles for 24 h (A) or 48 h (B). (C) Cell viability of AsPC-1 cells treated with blank micelles for 24 h and 48 h. (D) Drug release behavior of free GA and GA micelles in vitro. | |
Though GA micelles displayed good controlled released behavior, the cytotoxicity, apoptosis induction and cellular uptake in vitro of GA micelles should be explored for more convincing conclusion. MTT method was used to study the cytotoxicity of GA micelles and MPEG-P(CL-ran-TMC) copolymer. As presented in Fig. 2A, both free GA and GA micelles inhibited the cell viability with no obvious difference at 24 h. The half-maximal inhibitory concentration (IC50) of free GA was 0.36 ± 0.02 μg/mL, while the IC50 of GA micelles was 0.32 ± 0.01 μg/mL. As shown in Fig. 2B, at 48 h, the IC50 of free GA or GA micelles was 0.34 ± 0.01 μg/mL or 0.30 ± 0.01 μg/mL, respectively. The cytotoxicity of MPEG-P(CL-ran-TMC) copolymer was presented in Fig. 2C, even though the concentration of MPEG-P(CL-ran-TMC) copolymer was 500 μg/mL, the cell viability was up to 94.64% ± 2.22% at 24 h, and 97.40% ± 6.68% at 48 h. Overall, GA micelles kept the same cytotoxicity as free GA, and MPEG-P(CL-ran-TMC) copolymer showed little cytotoxicity to cells.
Apoptosis of cells treated with GA micelles was detected by the PI/Annexin V staining and analyzed by FCM. As shown in Fig. 3A and B, the apoptotic percentage of AsPC-1 cells treated with 0.3 μg/mL GA micelles was 50.14% ± 3.96%, while that in 0.3 μg/mL free GA group was 49.70% ± 3.93%. The apoptotic percentages of two groups were significantly higher than that of NS group (P < 0.05), however, the apoptotic percentage between free GA group and GA micelles group showed no significant difference.

|
Download:
|
| Fig. 3. (A) Apoptosis induction studies of free GA and GA micelles; (B) ratio of apoptosis in different groups (*P < 0.05); (C) cellular uptake studies of free GA and GA micelles (**P < 0.01). | |
The cellular uptake of free GA and GA micelles were tested by HPLC. As shown in Fig. 3C, the intracellular concentration of GA in free GA group was lower than that in GA micelles group at 2 h. Moreover, at 4 h, the intracellular concentration of GA in GA micelles group was significantly higher than that in free GA group (P < 0.01). Therefore, GA micelles enhanced the delivery of GA into AsPC-1 cells, and increased the cytotoxic efficacy of the drug. Therefore, GA micelles could effectively improve the cellular uptake of GA to AsPC-1 cells.
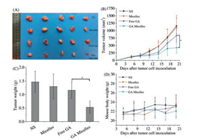
To further evaluate antitumor efficacy of GA micelles, in vivo subcutaneous xenograft AsPC-1 tumor model was used in this study. The photograph of tumors treated with NS, blank micelles, free GA, and GA micelles were shown in Fig. 4A. The tumors in GA micelles group were obviously smaller than that in free GA micelles group. As shown in Fig. 4B, the tumor volume of GA micelles group was controlled within a small size throughout the treatment process, and much smaller than that of free GA group at day 20 (P < 0.05). In addition, the tumor weight in GA micelles group was significantly less than that in free GA group at day 20 (Fig. 4C, P < 0.05). These results indicated that GA micelles had more effective inhibition on subcutaneous tumor growth. The mechanisms of GA micelles enhanced anti-tumor activity included these aspects: enhanced tumor cells apoptosis, more powerful suppression of tumor cells proliferation, and more GA accumulated at the tumor site due to the EPR effect. The nano-size and the presence of the hydrophilic shell of GA micelles may contributed to the EPR effect. The representative H & E staining images of tumor sections of four groups suggested that GA micelles obviously inhibited malignant proliferation of tumor, and the group treated with GA micelles had more signs of apoptosis, such as apoptotic body and karyopyknosis (Fig. S2 in Supporting information). It indicated that GA micelles showed better induction of apoptosis than free GA. Body weight of mice among groups showed no significant difference (Fig. 4D), which suggested that GA micelles had no obvious toxicity to mice. Complete blood count (CBC) and serum chemistry profile analysis of mice were shown in Figs. S3 and S4 in Supporting information, which illustrated no obvious difference among groups.
|
Download:
|
| Fig. 4. GA micelles inhibited tumor growth in subcutaneous AsPC-1 tumor model. (A) Representative photograph of subcutaneous tumors in each group; (B) Volume of subcutaneous tumors in each group (*P < 0.05); (C) Weight of subcutaneous tumors in each group (*P < 0.05); (D) Body weight of AsPC-1 tumor-bearing mice in each group. | |
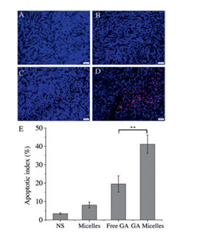
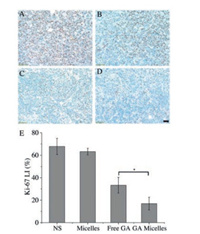
Moreover, to further demonstrate the mechanism of antitumor in vivo, histological analysis of the tumor sections was performed at the end of treatment. TUNEL immunofluorescent staining assay was adopted to measure apoptosis in free GA group and GA micelles group. As shown in Fig. 5, apoptosis index in GA micelles group (41.17%± 4.98%) wasmuch higher than thatoffreeGA group (19.54% ± 4.45%, P < 0.01). It indicated that GA micelles could effectively promote more tumor apoptosis than free GA. Immunohitochemical staining of Ki-67 was used to examine the proliferation activity of tumor tissues. As shown in Fig. 6, the Ki-67 of GA micelle group (16.96% ± 5.66%) was significantly lower than free GA group (33.34% ± 7.02%, P < 0.05). It indicated that the inhibition of tumor proliferation by GAmicelles was more powerful than that of free GA.
|
Download:
|
| Fig. 5. Representative TUNEL immunofluorescent images of tumor sections in (A) NS, (B) blank micelles, (C) free GA, and (D) GA micelles groups, and mean apoptotic index in each group (E) (**P < 0.01). Scale bar = 50 μm. | |
|
Download:
|
| Fig. 6. Representative Ki-67 immunohistochemichal images of tumor sections in (A) NS, (B) blank micelles, (C) free GA, and (D) GA micelles groups and mean Ki-67 LI in each group (E) (*P < 0.05). Scale bar = 50 μm. | |
In summary, GA encapsulated MPEG-P(CL-ran-TMC) polymer micelles were prepared and applied for therapy on AsPC-1 pancreatic cancer. It was demonstrated that GA micelles had significant inhibition on cell viability and apoptosis-inducing effect in vitro. Compared to free GA, GA micelles showed good water dispersion, delay-released behavior, and more cellular uptake. Moreover, GA micelles exerted more effective therapeutic effect and better apoptosis induced effect in a subcutaneous xenograft mouse model of AsPC-1 cells. In conclusion, GA micelles showed high-efficiency anti-tumor effect in vitro and in vivo, and they could serve as a candidate for pancreatic cancer treatment.
AcknowledgementsThis work was financially supported by National Natural Science Foundation of China (No. 81822025), National Program for Support of Top-notch Young Professionals (No. W02070141) and 1·3·5 Project for Disciplines of Excellence, West China Hospital, Sichuan University.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2019.02.018.
| [1] |
J. Ferlay, I. Soerjomataram, R. Dikshit, et al., Int. J. Cancer 136 (2015) E359-E386. DOI:10.1002/ijc.29210 |
| [2] |
J. Cullis, D. Siolas, A. Avanzi, et al., Cancer Immunol. Res. 5 (2017) 182-190. DOI:10.1158/2326-6066.CIR-16-0125 |
| [3] |
R.L. Siegel, K.D. Miller, A. Jemal, CA-Cancer J. Clin. 65 (2015) 5-29. DOI:10.3322/caac.21254 |
| [4] |
Y. Pang, C. Kartsonaki, Y. Guo, et al., Int. J. Cancer 140 (2017) 1781-1788. DOI:10.1002/ijc.30599 |
| [5] |
T. Kamisawa, L.D. Wood, T. Itoi, K. Takaori, Lancet 388 (2016) 73-85. DOI:10.1016/S0140-6736(16)00141-0 |
| [6] |
F.L. Besson, J.J. Parienti, B. Bienvenu, et al., Eur. J. Nucl. Med. Mol. Imaging 38 (2011) 1764-1772. DOI:10.1007/s00259-011-1830-0 |
| [7] |
C. Gong, C. Wang, Y. Wang, et al., Nanoscale 4 (2012) 3095-3104. DOI:10.1039/c2nr30278k |
| [8] |
A. Panthong, P. Norkaew, D. Kanjanapothi, et al., J. Ethnopharmacol. 111 (2007) 335-340. DOI:10.1016/j.jep.2006.11.038 |
| [9] |
T. Wang, J. Wei, X. Qian, et al., Cancer Lett. 262 (2008) 214-222. DOI:10.1016/j.canlet.2007.12.004 |
| [10] |
M. Ishaq, M.A. Khan, K. Sharma, et al., BBA-Gen. Subj. 1840 (2014) 3374-3384. DOI:10.1016/j.bbagen.2014.08.019 |
| [11] |
Y. Qu, G. Zhang, Y. Ji, et al., Phytomedicine 23 (2016) 350-358. DOI:10.1016/j.phymed.2016.01.011 |
| [12] |
V. Saxena, M.D. Hussain, Int. J. Nanomed. 2012 (2012) 713-721. |
| [13] |
H. Gu, S. Rao, J. Zhao, et al., J. Cancer Res. Clin. 135 (2009) 1777-1782. DOI:10.1007/s00432-009-0624-2 |
| [14] |
L. Zhao, Q.L. Guo, Q.D. You, et al., Biol. Pharm. Bull. 27 (2004) 998-1003. DOI:10.1248/bpb.27.998 |
| [15] |
W. Liu, Q.L. Guo, Q.D. You, et al., World J. Gastroenterol. 11 (2005) 3655-3659. DOI:10.3748/wjg.v11.i24.3655 |
| [16] |
T. Yi, Z. Yi, S.G. Cho, et al., Cancer Res. 68 (2008) 1843-1850. DOI:10.1158/0008-5472.CAN-07-5944 |
| [17] |
M. Youns, A. Elkhoely, R. Kamel, N-S Arch. Pharmacol. 391 (2018) 1-10. |
| [18] |
L.M. Saeed, M. Mahmood, S.J. Pyrek, et al., J. Appl. Toxicol. 34 (2014) 1188-1199. DOI:10.1002/jat.v34.11 |
| [19] |
F.Z. Dahmani, Y. Xiao, J. Zhang, et al., Adv. Healthc. Mater. 5 (2016) 1447-1461. DOI:10.1002/adhm.201600169 |
| [20] |
K. Hao, X.Q. Liu, G.J. Wang, et al., Eur. J. Drug Metab. Phram. 32 (2007) 63-68. DOI:10.1007/BF03190993 |
| [21] |
Y. Ji, S. Shan, M. He, et al., Acta Biomater. 62 (2017) 234-245. DOI:10.1016/j.actbio.2017.08.036 |
| [22] |
A.P. Ranjan, A. Mukerjee, A. Gdowski, et al., J. Biomed. Nanotechnol. 12 (2016) 679-688. DOI:10.1166/jbn.2016.2207 |
| [23] |
S.K. Verma, S. Rastogil, I. Arora, et al., J. Biomed. Nanotechnol. 12 (2016) 274-285. DOI:10.1166/jbn.2016.2153 |
| [24] |
Q. Wu, S. Deng, L. Li, et al., Nanoscale 5 (2013) 12480-12493. DOI:10.1039/c3nr04651f |
| [25] |
Q. Wu, G. Li, S. Deng, et al., Nanoscale 6 (2014) 11940-11952. DOI:10.1039/C4NR02978J |
| [26] |
L. Qiu, L. Zhao, C. Xing, Y. Zhan, Chin. Chem. Lett. 29 (2018) 301-304. DOI:10.1016/j.cclet.2017.09.048 |
| [27] |
C. Gong, Y. Xie, Q. Wu, et al., Nanoscale 4 (2012) 6004-6017. DOI:10.1039/c2nr31517c |
| [28] |
J. Wang, Q. Liu, L. Yang, et al., J. Biomed. Nanotechnol (2017) 1631-1646. |
| [29] |
Q. Fan, Z. Li, T. Hao, et al., Curr. Org. Chem. 20 (2016) 1835-1848. DOI:10.2174/1385272820666151228194438 |
| [30] |
S.H. Park, G.C. Bo, K.J. Min, et al., Macromolecules 41 (2008) 6486-6492. DOI:10.1021/ma800562s |
| [31] |
X. Yang, Z. Li, N. Wang, et al., Sci. Rep 5 (2015) 10322. DOI:10.1038/srep10322 |
 2019, Vol. 30
2019, Vol. 30 

