b Department of Chemistry, Lanzhou University, Lanzhou 730000, China
Deep eutectic solvents (DESs) have received rapidly increasing attention over the last few years as attractive alternatives to conventional ionic liquids [1-3]. These solvents are a class of compositionally various extent of low-transition-temperature mixtures and illustrate a set of intrinsically "designer solvents", which usually prepared by mixing of hydrogen-bonding acceptors (HBAs) and hydrogen-bonding donors (HBDs) species in the eutectic molar ratio. The physicochemical characteristics of DESs are respecting to those of ionic liquids and their compositions [4-6]. This unusual structure can be fixed by sorting of the molar ratio and molecular chemical moieties, and this extra dimension of design independence has provided the promotion of DESs as "greener" potential solvents for organic synthesis [7-10], extraction [11-15], separation [16-19], materials [20-22] and electrochemistry [23-25], implies attractive extensive scientific and technological interest as to ionic liquids (ILs) analog [26].
DESs have ultra-low vapor pressures at room temperature predominantly and also have significant benefits such as does not require any solvent and refining steps, and their minimal toxicity and cost, since the compounds used are generally non-toxic and abundant from renewable resources [27, 28]. Up to now, all the reported DESs emerged on a mixture of quaternary ammonium or phosphonium-based salts with a diversity of hydrogen bond donors [29-32].
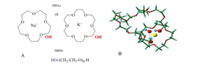
Herein, we report for the first time to synthesize a new family of designer solvents formed by crown ether (CE) complex as HBAs and polyethylene glycol (PEG) as HBDs (Scheme 1A). Furthermore, the structure of DES was simulated using 15-crown-5 ether, NaOH, and PEG by using PEG-458 (n = 10) as a representative of PEG polymer as shown in Scheme 1B. It was found that PEG was bent to form a hydrogen bond with the hydroxide anion of NaOH. The bond length of two H…O-H bonds in DES was 1.850 and 1.681 Å, respectively. Furthermore, Na+ was coordinated into the electronhole of 15-crown-5 ether. The results showed that the three compounds were intimately connected by the electrostatic and hydrogen-bonding interactions to form a very stable basic DES. This is the specialty of CE-based DESs, to make them different from other existing common DESs. These DESs show unique physical properties, which enables ultra-deep extraction of non-basic N-compounds as well as superior regeneration performance to set a new benchmark for N-compounds extraction by solvents.
|
Download:
|
| Scheme 1. The structures of HBAs and HBDs (A), and Schematic representation of the DES structure performed by MD simulation (B) (C green, Na+ yellow, O red, H cyan). | |
CE complex with sodium or potassium hydroxide (Na+-15- crown-5 ether or K+-18-crown-6 ether) were used as HBAs, low molecular PEG including PEG-200, PEG-400 and PEG-600 was chosen as HBDs. The DESs were formed by direct heating the mixtures of CE, hydroxide and PEG at an appropriate molar ratio. To reduce the cost and enhance the economy of application of these DESs, we used a higher molar ratio of PEG to explore whether it is formed DESs with small amount of CE complex since CE is expensive. It was observed that approximately 0.83% CE was sufficient to produce very stable DESs with 100% yield when using 44 molar ratio of PEG. The hydroxide (OH-) acceptor of crown ether complex rapidly induced to form DESs with the donor of PEG through the hydrogen-bonding interactions. PEG as the main compositon has numerous attractive properties including high boiling point, ultra-low vapor pressure, low toxicity as well as low cost, which makes these new DESs are green and cheap.
Compared with commonly used ammonium and phosphonium DESs, CE complex-based DESs is a new kind of basic solvents. The physical properties of these DESs were determined as listed in Table 1. All the DESs have low viscosity (cP), melting point (Tm) and freezing point (Tf), which indicated desirable characteristics of these DESs. Moreover, the good linear relationship was observed between the viscosity (cP) and the conductivity (μS/cm), where the conductivity increased with the decreasing of the viscosity, and the conductivity was 4-57 times higher than all the reported choline chloride-based common DESs due to the presence of metal ions in CE-DESs [33], which also signifying the anomalous behavior of these DESs. Furthermore, the HBDs mainly influenced on the viscosity of DESs, where PEG-600 based DESs showed little higher viscosity compared to PEG-200 and PEG-400-based DESs. This could be due to a longer oxygen-containing polymer chain of PEG- 600. More characterizations to confirm the structure of DESs including FT-IR and 1H NMR can be found in Figs. S1 and S2 in Supporting information.
|
|
Table 1 Compositions of new DESs and their physical properties at 298 K. |
The physical properties demonstrate that CE-DESs are lowviscosity solvents, with low-melting points and freezing points. Therefore, it is expected that they can have a promising applications in the future. To test this prediction, we investigated the extraction of carbazole, indole, and pyrrole from model fuel oils using CE-DESs as extractants, and compared with the commonly used DESs, since DESs can serve as alternative solvents in the liquid-liquid extraction of solutes from non-polar solvents.
Recently, the extraction of N-compounds from fuel oils was studied by using common DESs [34, 35]. Nevertheless, the fruitful extraction of non-basic N-compounds is still questionable. Typically, the essence of non-basic N-compounds in fuel oils are much higher (~70%) than basic one, and, hence the effect of nonbasic N-compounds on hydrodesulfurization (HDS) catalytic activity could become more significant [36]. In previous studies, acidic extractants or adsorbents, e.g., liquid acids, metal ion-loaded solvents and framework materials could efficiently extract basic N-compounds from fuels. However, their affinity to non-basic N-compounds was much lower [37-39]. Thus, it is still very critical importance to the ultra-deep separation of non-basic N-compounds by environment-friendly appropriate extractants and process due to their lower reactivity in hydrodenitrogenation, potent inhibitors of hydrodesulfurization process, and potential adsorption capacity on the surface of the catalyst [40-48].
Fig. 1 shows the distribution coefficients (D) of carbazole, indole, and pyrrole. Interestingly, it was found that these CE-based DESs have proven to be excellent extractants for N-compounds, and the results indicate that CE-DESs were more efficient for the extraction of non-basic nitrogen compounds than choline chloride (ChCl)-based common DESs. The D values of carbazole, indole, and pyrrole were below 100 when using ChCl-based DESs as extractants. Nevertheless, surprisingly, when the CE-DESs were applied, unprecedented D values of carbazole, indole, and pyrrole were obtained. The lower removing of carbazole, indole, and pyrrole by ChCl-based DESs indicated the importance of the structure of HBAs component of the DESs. ChCl in common DESs is much weaker basic HBA than CE-complex, hence leading to poorer extraction of non-basic N-compounds than CE-based DESs. However, when PEG-600 was used as HBD with ChCl, the D values of carbazole, indole, and pyrrole slightly increased.

|
Download:
|
| Fig. 1. Distribution coefficient of carbazole (black), indole (orange), pyrrole (green), with common DESs and CE-DESs at 308 K with a 1: 1 mass ratio of extractant:oil. A. ChCl +1, 2-propanediol, B. ChCl + Resorcinol, C. ChCl + Ethylene glycol, D. ChCl + PEG- 600, E. DES1, F. DES2, G. DES3, H. DES4, I. DES5, J. DES6. | |
On the other hand, the details of denitrogenation performance using CE-DESs at 308 K, with a 1:1 mass ratio of DESs to oil were investigated as shown in Table S1 (Supporting information). All in all, DES3 and DES6 were more powerful during the experimental process and exhibited ultra-deep separation of non-basic N-compounds. The results indicate that PEG-600 was more effective HBD in both common and CE-DESs, because it has longer oxygen-containing polymer chain including loan pair of electrons in its oxygen atom, compared with PEG-200 and PEG-400, which also can matter to enhance the extraction capability of hydrogen-bond donor molecules like carbazole, indole, and pyrrole. Moreover, CE-DESs have exceptional chemical structure than common DESs where the HBAs belong to alkali metal ions (Na+ and K+), which increase the basicity and probably taking part in the strong cationπ interactions (non-covalent molecular interaction) with aromatic π-electron of non-basic N-compounds, because alkali metal cations have strong tendency to interact with the face of an electron-rich π system of the compounds.
Besides, the excessive basicity of HBA and HBD in CE-DESs probably also command the very favored extraction of non-basic N-compounds. Because higher basicity of DESs components probably caused a weaker "internal" HBA-HBD interaction and thus strongest the "external" HBA ability of the DES to extract the hydrogen-bond acidic molecules through the strong H-bonding interactions. However, the CE-DESs show the weaker "external" HBA ability toward basic N-compounds, which may due to the firm basic character of DESs components, thus shows a feeble interaction with quinoline and pyridine. Moreover, the basic N-compounds are not comprised with the aromatic system, thus these compounds not involved in the cation-π interactions. As a consequence, the lower D and E values were obtained (Table S1).
An ideal denitrogenation extractant might have not only optimum extraction efficiency of N-compounds but also low solubility in oils to gain large-scale nitrogen-to-oil selectivity. Fig. S3 (Supporting information) shows the solubility of n-heptane in different DESs at 308 K. As seen from Fig. S3, the solubility of DES3 and DES6 were 30.3 mg/g and 38.5 mg/g, little higher than other DESs. However, these two DESs exhibited excellent selectivity for carbazole, indole, and pyrrole simultaneously as compared to others is shown in Fig. 2. It was observed that DES3 only had little higher selectivity to pyrrole due to the lower solubility with n-heptane than DES6. We can say that DES3 showed the optimum extraction efficiency and the excellent selectivity of N-compounds to n-heptane, which is extremely attractive for the denitrogenation of fuel oils. Therefore, considering the overall extraction ability of all DESs, DES3 can be regarded as the best representative extractant on the denitrogenation of the Ncontaining fuel oils. Hence, several essential parameters including the effect of mass ratio, temperature, time, initial concentration, and regeneration were investigated using DES3 as the extractant for more details information on this extraction process (Figs. S4–S9 in Supporting informaiton).

|
Download:
|
| Fig. 2. Selectivity of carbazole to n-heptane (black), indole to n-heptane (orange) and pyrrole to n-heptane (green) by different DESs at 308 K and a DES: oil mass ratio of 1:1. | |
On the other hand, a practical denitrogenation experiment was carried out with real gasoline using DES3 as extractant. From the HPLC analysis, it was found that many compounds were extracted, and among them the peak of carbazole and indole were comfirmed as shown in Fig. S10 (Supporting informaiton), indicating an excellent extractability of CE-DESs even for real fuel oils, which is desirable from the point of view of an industrial process.
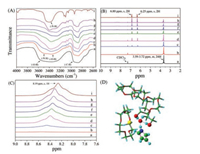
The DESs could separate non-basic N-compounds efficiently from fuel oils, and hence the extraction mechanism can be extremely important to know the denitrogenation process although it is a challenge. Since the apparent extraction mechanism of N-compounds using DESs has not yet been investigated or reported. To prove our hypothesis, DES3 and pyrrole were taken as a typical example to investigate the mechanism using FT-IR, 1H NMR, and MD simulation systematically. Fig. 3A shows the FT-IR result of different mass ratios between DES3 and pyrrole. The -OH broad absorption band was observed at 3362 cm-1 in DES3, while the -NH stretching vibration in pyrrole was observed at 3400 cm-1. With increasing the concentration of pyrrole in DES, the external HBA capability of DES with -NH proton of pyrrole becomes stronger. This can be supposed from the peak of the -OH and -NH group was overlapped in the extracted species, and the overlap was obviated with increasing the mass ratio. This change in the stretching band is mainly due to the mutation in the different vibrational atmosphere of the bonds in molecules, and a part of the electron cloud in a nitrogen atom migrated to lower wavenumbers. These results suggested the presence of hydrogen-bonding between OH- of HBA in DES3 and -NH group of pyrrole.
|
Download:
|
| Fig. 3. FT-IR (A), 1H NMR (B) and the enlarged part of 1H NMR from δ 7.6 to δ 9.0 (C) of different mass ratios (DES3 : Pyrrole). (a) 1:0, (b) 1:0.1, (c) 1:0.5, (d) 1:1, (e) 1:1.5, (f) 1:2, (g) 1:3, (h) 1:4, (i) 0:1, and MD simulation calculated binding site of pyrrole (N blue, O red, H cyan) with DES (D), H-bonds pink broken lines. | |
To further examine the mechanism, 1H NMR was performed as shown in Fig. 3B. The multiple H signals at δ 3.59-3.72 are assigned to the -CH2-CH2, a group of PEG and CE in DES3. Furthermore, in the extracted species, the proton signal of pyrrole was obviated, and intensity of the peak increased with the mass ratios of pyrrole. The two identical H singlet peaks of net pyrrole were observed at δ 6.25 and δ 6.80, while the corresponding singlet signal appeared at the same position in the extracted species, which pointed out that the charge density of the proton remained unvaried while the reaction was proceeding. Moreover, the broad singlet was observed at δ 8.19 of -NH group in pyrrole shifted to the lower field region in the extracted species gradually as shown in Fig. 3C. This also confirms that the active hydrogen-bonding interaction was formed between OH- of HBA in DES3 and -NH group of pyrrole.
The mechanism was also investigated by the molecular dynamic (MD) simulation. Under the same condition, pyrrole was used as a representative N-compound into the system to investigate the interaction between DES and non-basic N-compounds is shown in Fig. 3D. We can see the hydrogen-bonding was formed between -N-H group of pyrrole and OH- anion of HBA of the DES, and the bond length is 1.629 Å. This result indicates the strong interaction of pyrrole with DES; hence the ultra-highseparation of non-basic N-compounds from fuel oils can be obtained using these DESs.
In summary, we synthesized a family of new DESs based on crown-ether HBAs and different PEG molecules as HBDs, which were used for highly efficient extraction of N-compounds from fuel oils. The exquisite structure and precise selection of HBAs and HBDs in DESs showed an intensive effect on the extraction of nonbasic N-compounds. Among the investigated DESs, DES3 presented the best denitrogenation performance. Without chemical reactions, the extraction efficiencies for carbazole, indole, and pyrrole were ~100%, while the distribution coefficients of specific solutes reached unprecedented values (~+∞) as well as exhibited record selectivity from model fuel oils, which distinguishes the superiority of these new materials. Finally, the denitrogenation performance of CE-DESs for real gasoline was also investigated. This work not only demonstrates the illustrious potential to introduce a new member of DESs family for ultra-high N-compounds extraction but also provides significant indications for other promising applications in chemistry and materials science.
AcknowledgmentsThis work was supported by the National Natrual Sciece Foundation of China (Nos. 21650110454, 21675164, 21822407), the CAS-President International Fellowship Initiative (No. 2017PC0014) and the Funds for Distinguished Young Scientists of Gansu (No. 1506RJDA281).
| [1] |
A.P. Abbott, G. Capper, D.L. Davies, R.K. Rasheed, V Tambyrajah, Chem. Commun (2003) 70-71. |
| [2] |
A.P. Abbott, D. Boothby, G. Capper, D.L. Davies, R.K. Rasheed, J. Am. Chem. Soc. 126 (2004) 9142-9147. DOI:10.1021/ja048266j |
| [3] |
A.P. Abbott, R.C. Harris, K.S. Ryder, et al., Green Chem. 13 (2011) 82-90. DOI:10.1039/C0GC00395F |
| [4] |
Y.T. Liu, Y.A. Chen, Y.J. Xing, Chin. Chem. Lett. 25 (2014) 104-106. DOI:10.1016/j.cclet.2013.09.004 |
| [5] |
E.L. Smith, A.P. Abbott, K.S. Ryder, Chem. Rev. 114 (2014) 11060-11082. DOI:10.1021/cr300162p |
| [6] |
W. Zhu, C. Wang, H. Li, et al., Green Chem. 17 (2015) 2464-2472. DOI:10.1039/C4GC02425G |
| [7] |
S. Gore, S. Baskaran, B. Konig, Green Chem. 13 (2011) 1009-1013. DOI:10.1039/c1gc00009h |
| [8] |
Z.H. Zhang, X.N. Zhang, L.P. Mo, Y.X. Li, F.P. Ma, Green Chem. 14 (2012) 1502-1506. DOI:10.1039/c2gc35258c |
| [9] |
M. Avalos, R. Babiano, P. Cintas, J.L. Jimenez, J.C. Palacios, Angew. Chem. Int. Ed. 45 (2006) 3904-3908. |
| [10] |
C.M. Clouthier, J.N. Pelletier, Chem. Soc. Rev. 41 (2012) 1585-1605. DOI:10.1039/c2cs15286j |
| [11] |
T. Gu, M. Zhang, T. Tan, et al., Chem. Commun. 50 (2014) 11749-11752. DOI:10.1039/C4CC04661G |
| [12] |
T. Tan, Z. Li, X. Mao, Y. Wan, H. Qiu, Anal. Methods 8 (2016) 3511-3516. DOI:10.1039/C6AY00053C |
| [13] |
Y. Dai, G.J. Witkamp, R. Verpoorte, Y.H. Choi, Anal. Chem. 85 (2013) 6272-6278. DOI:10.1021/ac400432p |
| [14] |
Y. Dai, J.V. Spronsen, G.J. Witkamp, R. Verpoorte, Y.H. Choi, J. Nat. Prod. 76 (2013) 2162-2173. DOI:10.1021/np400051w |
| [15] |
M.W. Nam, J. Zhao, M.S. Lee, J.H. Jeong, J. Lee, Green Chem. 17 (2015) 1718-1727. DOI:10.1039/C4GC01556H |
| [16] |
T. Gu, M. Zhang, J. Chen, H. Qiu, Chem. Commun. 51 (2015) 9825-9828. DOI:10.1039/C5CC02553B |
| [17] |
B. Tang, H. Zhang, K.H. Row, J. Sep. Sci. 38 (2015) 1053-1064. DOI:10.1002/jssc.201401347 |
| [18] |
A. Shishov, A. Bulatov, M. Locatelli, S. Carradori, V. Andruch, Microchem. J. 135 (2017) 33-38. DOI:10.1016/j.microc.2017.07.015 |
| [19] |
B. Yang, T. Cai, Z. Li, M. Guan, H. Qiu, Talanta 175 (2017) 256-263. DOI:10.1016/j.talanta.2017.07.038 |
| [20] |
E.R. Parnham, E.A. Drylie, P.S. Wheatley, A.M.Z. Slawin, R.E. Morris, Angew. Chem. Int. Ed. 45 (2006) 4962-4966. |
| [21] |
L. Vidal, C. Marichal, V. Gramlich, J. Patarin, Z. Gabelica, Chem. Mater. 11 (1999) 2728-2736. DOI:10.1021/cm991027o |
| [22] |
L. Liu, J. Yang, J. Li, et al., Angew. Chem. Int. Ed. 50 (2011) 8139-8142. DOI:10.1002/anie.v50.35 |
| [23] |
C. Zhang, Y. Ding, L. Zhang, et al., Angew. Chem. Int. Ed. 56 (2017) 7454-7459. DOI:10.1002/anie.201703399 |
| [24] |
H. Liao, Y. Jiang, Z. Zhou, S. Chen, S. Sun, Angew. Chem. 120 (2008) 9240-9243. DOI:10.1002/ange.v120:47 |
| [25] |
A.P. Abbott, G. Capper, K.J. Mckenzie, K.S. Ryder, J. Electroanal. Chem. 599 (2007) 288-294. DOI:10.1016/j.jelechem.2006.04.024 |
| [26] |
C. Florindo, F.S. Oliveria, L.P.N. Rebelo, A.M. Fernandes, I.M. Marrucho, ACS Sustain. Chem. Eng. 2 (2014) 2416-2425. DOI:10.1021/sc500439w |
| [27] |
L.X. Zhang, H. Yu, H.B. Yu, Z. Chen, L. Yang, Chin. Chem. Lett. 25 (2014) 1132-1136. DOI:10.1016/j.cclet.2014.03.029 |
| [28] |
M. Francisco, A. van den Bruinhorst, M.C. Kroon, Angew. Chem. Int. Ed. 52 (2013) 3074-3085. DOI:10.1002/anie.201207548 |
| [29] |
K. Pang, Y.C. Hou, W.Z. Wu, W.J. Guo, W. Peng, Green Chem. 14 (2012) 2398-2401. DOI:10.1039/c2gc35400d |
| [30] |
T. Tan, M. Zhang, Y. Wan, H. Qiu, Talanta 149 (2016) 85-90. DOI:10.1016/j.talanta.2015.11.041 |
| [31] |
H. Zhang, X. Qiao, T. Cai, et al., Anal. Bioanal. Chem. 409 (2017) 2401-2410. DOI:10.1007/s00216-017-0187-z |
| [32] |
P.K. Naik, S. Paul, T. Banerjee, J. Mol. Liq. 243 (2017) 542-552. DOI:10.1016/j.molliq.2017.08.044 |
| [33] |
Q. Zhang, K.D.O. Vigier, S. Royer, F. Jérôme, Chem. Soc. Rev. 41 (2012) 7108-7146. DOI:10.1039/c2cs35178a |
| [34] |
M.C. Ali, Q. Yang, A.A. Fine, et al., Green Chem. 18 (2016) 157-164. DOI:10.1039/C5GC01823D |
| [35] |
H.F. Hizaddin, M.K. Hadj-Kali, A. Ramalingam, M.A. Hashim, J. Chem. Thermodyn. 95 (2016) 164-173. DOI:10.1016/j.jct.2015.12.009 |
| [36] |
U.T. Turaga, G. Wang, X. Ma, C. Song, Prepr. Pap.-Am. Chem. Soc. Div. Fuel Chem. 48 (2003) 550-552. |
| [37] |
I. Ahmed, N.A. Khan, S.H. Jhung, Inorg. Chem. 52 (2013) 14155-14161. DOI:10.1021/ic402012d |
| [38] |
S. Kumar, V.C. Srivastava, S.M. Nanoti, A. Kumar, AIChE J. 61 (2015) 2257-2267. DOI:10.1002/aic.14809 |
| [39] |
G.H.C. Prado, Y. Rao, A. Klerk, Energy Fuels 31 (2017) 14-36. DOI:10.1021/acs.energyfuels.6b02779 |
| [40] |
X.D. Tang, T. Hu, J.J. Li, Petro. Technol. 43 (2014) 843-847. |
| [41] |
Z. Zhou, W. Li, J. Li, Chem. Biochem. Eng. Q. 31 (2017) 63-68. DOI:10.15255/CABEQ |
| [42] |
J. Zhang, J. Xu, J. Qian, Petrol. Sci. Technol. 31 (2013) 777-782. DOI:10.1080/10916466.2010.493911 |
| [43] |
L. Zhang, D. Xu, J. Gao, et al., Fuel 194 (2017) 27-35. DOI:10.1016/j.fuel.2016.12.095 |
| [44] |
A. Marafi, A. Stanislaus, E. Furimsky, Catal. Rev. 52 (2010) 204-324. DOI:10.1080/01614941003720167 |
| [45] |
D.Y. Han, G.X. Li, Z.B. Cao, X.Y. Zhai, M.M. Yuan, Energ. Source Part A 35 (2013) 622-628. DOI:10.1080/15567036.2010.509085 |
| [46] |
I. Ahmed, S.H. Jhung, Chem. -Eng. J. 279 (2015) 327-334. DOI:10.1016/j.cej.2015.05.035 |
| [47] |
M.R. Shah, R. Anantharaj, T. Banerje, G.D. Yadav, J. Chem. Thermodyn. 62 (2013) 142-150. DOI:10.1016/j.jct.2013.02.020 |
| [48] |
M. Vilas, E.J. Gonzalez, E. Tojo, Fluid Phase Equilib. 396 (2015) 66-73. DOI:10.1016/j.fluid.2015.03.032 |
 2019, Vol. 30
2019, Vol. 30 


