b CAS Key Laboratory of Chemistry of Northwestern Plant Resources and Key Laboratory for Natural Medicine of Gansu Province, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China
High performance liquid chromatography (HPLC), as one of the most commonly-used analysis methods [1-3], has been nearly penetrated every chemical applications. Continuous improvement on chromatographic performance has been made and the subject is the direction of ongoing research. Hence, a wide variety of HPLC packing materials with specific selectivities are available. With the in-depth research of chromatographic separation performance, the commonly used reversed-phase liquid chromatography (RPLC) and normal phase liquid chromatography (NPLC) are not suitable for the very polar and hydrophilic samples. As a complementary alternative, hydrophilic interaction chromatography (HILIC) integrates the advantages of both NPLC and RPLC. Besides, HILIC is widely used as a general-purpose model in which various functional groups are introduced. Up to now, researchers has made great developments for designation of HILIC stationary phases such as amide [4], saccharides [5], zwitterionic molecules [6], organic polymers or inorganic oxides carrying various polar groups [7-10], which provide appropriate separation of polar pharmaceuticals, carbohydrates, peptide, proteins and oligosaccharides [11, 12]. The appearance of carbon nanomaterials has also opened a window for developing new generation HILIC columns. Some carbon nanomaterials with hydrophilic functional groups like graphene oxide (GO) [13], fullerenes [14], oxidized nanodiamond [15], and carbon nanoparticle (CNP) [16] were selected to prepare HILIC stationary phases, which showed acceptable separation performance.
In particular, as promising carbonaceous nanomaterials, the graphene-based family are promising to make an excellent contribution to the development of novel HPLC stationary phases. Since Novoselov et al. directly observed and characterized graphene in 2004, there has been a keen surge attraction in graphene because of its particular structure and property advantages [17]. However, in practical applications, the recognition of its potential has been severely limited by the strong van der Waals interactions and violent π-π interaction between graphene singlelayer structures. It is very difficult to gain a stable graphene structure on materials surfaces. Subsequently, graphene oxide (GO) has overcame this problem because of the abundant oxygenous groups (such as epoxy groups, hydroxyl groups and carboxyl groups) on the edge and surface of the sheets [18, 19]. To date, there are only several reports on the application of the graphene family including graphene [20, 21], graphene oxide [22-24] and graphene quantum dots [25] as HPLC stationary phases. The main reason is that graphene nanosheets do not form a uniform and stable separation matrix when directly filled into an HPLC column. Moreover, the irregular shape of the nanosheets should greatly reduce the efficiency of the column [26].
Simultaneously, porous graphene (PG) as another graphene-based material has also attracted increasing interest recently. Generally, PG can be described as a collection of graphene-based materials with some random or high regular carbon atoms vacancies in the plane. According to the production techniques used, the pore size can be categorized from atomic precision to nanoscale, as micropores (diameters below 2 nm), mesopores in the range 2–50 nm, and macropores for diameters above 50 nm [27, 28]. On account of the nanopores in the graphene sheet, this material exhibits potential properties different from pristine graphene material, introduced its latent applications in multitudinous fields such as DNA sequencing [29], energy storage [30], and gas purification [31]. The pores in the porous structures with different dimensions and shapes have caused them to be outstanding and remarkable for various applications. When applied to chromatographic packing materials, the shape and size of the pores can participate in the absorption and partitioning of the analytes according to the size of cavities in the stationary phase. This can compensate for the inability of the graphene nanosheets to form a uniform and stable separation matrix when filled into an HPLC column. To its credit, our research group has independently developed a special local combustion method to prepare PG from graphene oxide [32]. Based on this, a facile synthesis scheme was mentioned for the manufacture of porous graphene-decorated spherical porous silica by using DESs as reaction media. Characterizations indicate that PG has been functionalized onto the surface of silica microspheres. Chromatographic performance of porous graphene-decorated silica column (Sil-PG) was evaluated by the separation of sulfonamides in HILIC mode. Compared with bare SiO2 column and a commercial HILIC column (Inspire 5 μm Hilic), this new stationary phase shows both hydrophilic and significant π-π interactions with sulfonamides analytes.
In this paper, PG was obtained by the local combustion method of graphene oxide. After that, the aminopropylated silica (Sil-NH2) wassynthesized via thegraftingof (3-aminopropyl)triethoxysilane on the silica. After mixing PG with Sil-NH2 and stirring in DESs, PG was coated on Sil-NH2 via strong electrostatic interaction. Thus, PG-modified silica (Sil-PG) was obtained as shown in Fig. 1. Other details can be found in Supporting information.
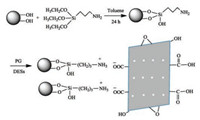
|
Download:
|
| Fig. 1. Schematic diagram for preparation of Sil-PG. | |
Transmission electron microscopy (TEM) was used to characterize the morphological property of as-synthesized PG and Sil-PG materials. The PG particles were well mono-dispersed and the uniform size with an average diameter of about 4.33 nm (Fig. 2a). Moreover, the PG nanoparticles were uniformly coated on the surface of spherical silica, as displayed in Fig. 2b. From the infrared spectrum of PG, Sil-NH2 and Sil-PG in Fig. 2c, we can see that the wavenumber at 2961 cm-1 was distributed to the asymmetric stretching of CH2; the signal at 1680 cm-1 was attributed to inplane deformation vibration of C=O and C=C from PG; the band around 1095 cm-1 was assigned to the stretching vibration of Si–O bond of silica or silylant agent. All of this preliminary indicates that the porous graphene group is successfully bonded to the silica. According to the analysis of XRD shown in Fig. 2d, the porous graphene has a broad and obvious diffraction peak around 23°, which was the same as the diffraction peak position of the graphene reported in the related literature [33]. Compared with PG, the Sil-PG also has a diffraction peak around 23°, and the peak shape and peak intensity are almost identical to those of PG. Some other characterizations including elemental analysis, SEM, Raman spectrum and TGA analyses also canprove that the synthesis of PGmodified silica materials is successful, which can be found in the Supporting information (Table S1, Figs. S1-S3 in Supporting information).

|
Download:
|
| Fig. 2. TEM image of PG, (b) Sil-PG; (c) FT-IR spectra of Sil-PG and (d) XRD pattern of Sil-PG. | |
The chromatographic performance of the prepared Sil-PG stationary phase was investigated by six sulfonamides samples. As shown in Fig. 3a, compared with the bare silica column and a commercial HILIC column (Inspire 5 μm Hilic), it appears that the six sulfonamides compounds can be baseline separated on the Sil-PG column within 10 min. Results showed that sulfanilamide had a weak retention, while sulfamethoxazole had a strong retention. It is known that porous graphene partially reduced chemically from GO, still contains some polar oxygen functional groups with a complete delocalized π–electron system. As shown in the results which can be explained by the mechanism that except for hydrophilic interaction, PG can also interact with analytes through π-π stacking interaction due to its highly delocalized conjugate system of π-electron on the surface.

|
Download:
|
| Fig. 3. (a) Chromatograms of sulfonamides were separated on Sil-PG, Inspire 5 μm Hilic and Silica columns: 1. sulfanilamide, 2. sulfamethazine, 3. sulfamerazine, 4. sulfasalazine, 5. sulfadiazine, 6. sulfamethoxazole. Mobile phase: acetonitrile-20 mmol/L aqueous ammonium acetate solution (85/15, v/v), wavelength: 254 nm, Flow rate: 1.0 mL/min, column temperature: room temperature. Effect of (b) volume fraction of water, (c) buffer concentration, and (d) pH on the retention factor (k) of six sulfonamides on Sil-PG. Mobile phase: acetonitrile-ammonium acetate solution (90:10, v/v), wavelength: 254 nm, Flow rate: 1.0 mL/min, column temperature: room temperature; the buffer concentration of (b) and (d) were 20 mmol/L, the buffer pH of (b) and (c) were 6.62. | |
To further evaluate the chromatographic properties, a set of chromatographic factors were studied to acquire the retention performance of the Sil-PG stationary phase. Firstly, the retention change (logk) with the logarithm of volume fraction of water in the mobile phase on sulfonamides was investigated as presented in Fig. 3b. The retention factors of six sulfonamides decreased gradually with the increase of water content, corresponding with the classic HILIC retention behavior. In typical HILIC models, the attached polar groups of stationary phase attract water molecules forming an aqueous layer over the surface and dissolved polar analytes in the mobile phase undergoes partitioning between semi-immobilized aqueous layer and the mobile phase [34]. The water-rich layer might not be adequate to sustain strong hydrophilic partitioning, so direct interactions between the solutes and stationary phases are more plausible at lower water content, and the phenomenon of Fig. 3b is consistent with this theory.
Secondly, the effectof buffer salt contentin the mobile phase on sulfonamides retention was investigated ranging from 10 mmol/L to 150 mmol/L (Fig. 3c). It is clear that the retention of sulfonamides on Sil-PG decreased with the increase of salt content, expressing that the electrostatic interaction with carboxyl on PG played the mainly role for the retention of samples. When buffer concentration getting higher, it suppressed the electrostatic attraction between solutes and stationary phase, and the retention of analytes getting weaker. Generally, as the salt concentration of mobile phase increased, more solvated salt ions were drived into the water-enriched layer and the water-rich layer became thicker. Simultaneously, the adsorbed water molecules inhibit the ionization of the surface charge [35]. The electrostatic interactions between charged solutes and ionic analytes decreased with the buffer concentration of mobile phase increased, and the retention of analytes reduced.
Moreover, mobile phase pH also plays a significant part in affecting selectivity in HILIC mode since it can affect the charge state of the stationary phase and polar solutes. In the HILIC mode, the charged solutes are more hydrophilic than the neutral form and therefore have a stronger retention. The protonated acid was less hydrophilic than the deprotonated form. When at low pH values, the proportion of protonated acid increased, leading to reduced electrostatic attraction and retention. Higher mobile phase pH in deprotonation results in more negatively charged species results, resulting in stronger retention [36]. All these conclusion were accordant with the experimental results that the retention of most sulfonamides increase with the growth of pH values from 3.5 to 7.5 (Fig. 3d). Additionally, the charge state of sulfonamide was substantially unaffected by the mobile phase pH, therefore the retention remaines almost constant within the pH range used in the study. The repeatability of PG bonded stationary phase was tested by continuous injection of the six sulfonamides during the intraday (Fig. S4 in Supporting information) and interday (Table S2 in Supporting information).
In the practical applications, sulfonamides are a series of synthetic antibacterial drugs with the advantages of broad antibacterial spectrum, stable properties and ease of use, which has been used clinically for nearly 50 years [37]. Moreover, sulfonamides can inhibit multiplication of most gram-positive and many gram-negative organisms and have been implicated in the growing prevalence of antibiotic resistance in humans. Therefore, it is very important for pharmacological study and quality control of sulfonamides products. In this work, the prepared Sil-PG column was utilized to analyze the sulfonamides in human serum sample. Acetonitrile (2 mL) was added to 0.5 mL of fresh serum in a stoppered test tube. Then the mixture was shaked for 10 min, centrifuged, and filtered with microporous membrane. The freshly treated filtrate was obtained for chromatographic analysis. In order to check the suitability of the resulted column, samples of sulfadiazine, sulfamethazine, and sulfisoxazole were spiked at six concentration levels (1, 10, 20, 30, 40, 50 μg/mL). The representative chromatograms of the human serum sample, standard solution of sulfadiazine, sulfamethazine, sulfisoxazole, and human serum sample spiked with standard solution via the prepared Sil-PG column were shown in Fig. 4a. With 90% ACN and 10% 20 μmol/L ammonium acetate solution as the mobile phase, there were no sulfonamide peaks in the human serum sample. When standard solution was added, the peaks of sulfadiazine, sulfamethazine and sulfisoxazole were well separated with the matrix of human serum sample. On the basis of the matrix addition method, standard curves of sulfadiazine, sulfamethazine and sulfisoxazole were obtained with good correlation coefficients (Fig. 4b). As shown in Table S3 (Supporting information), good recoveries and RSDs of human serum sample demonstrated that the prepared Sil-PG stationary phase exhibited equivalent potential for applications in the field of pharmaceutical analysis.

|
Download:
|
| Fig. 4. (a) Human serum sample, standard solution with a concentration of 0.1 mg/mL, and human serum samples spiked with standard solutions of sulfadiazine (1), sulfamethazine (2) and sulfisoxazole (3). (b) Standard curves of sulfadiazine (1), sulfamethazine (2) and sulfisoxazole (3). Mobile phase: acetonitrile-20 mmol/L aqueous ammonium acetate solution (90:10, v/v), wavelength: 254 nm, flow rate: 1.0 mL/min, room temperature. | |
In summary, a novel Sil-PG stationary phase was successfully synthesized through assembling porous graphene on silica microspheres. Characterizations of the obtained chromatographic materials verified that PG was successfully coated on the surface of silica microspheres. The obtained column demonstrated complicated chromatographic performance owing to the primary retention mechanisms containing π-π stacking interaction, electrostatic interaction and partitioning. Compared with bare SiO2 column, the Sil-PG column could achieve effective separation of six sulfanilamide compounds within a reasonable elution time. Moreover, the Sil-PG column was applied for detection of sulfanilamides in human serum samples with satisfying results. Notably, the new Sil-PG stationary phase offers the possibility to broaden the application of PG in the field of chromatography, and the utility of the Sil-PG composites in various functional applications will be further stretched in future.
AcknowledgmentsFinancial supports from the National Natural Science Foundation of China (Nos. 21822407 and 21675164) and the funds for Distinguished Young Scientists of Gansu (No. 1506RJDA281) are gratefully acknowledged.
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.10.040.
| [1] |
A.K. Mallik, H. Qiu, T. Oishi, et al., Anal. Chem. 87 (2015) 6614-6621. DOI:10.1021/acs.analchem.5b00663 |
| [2] |
M. Zhang, W. Mai, L. Zhao, Y. Guo, H. Qiu, J. Chromatogr. A 1388 (2015) 133-140. DOI:10.1016/j.chroma.2015.02.023 |
| [3] |
A.K. Mallik, H. Qiu, Y. Kuwahara, M. Takafuji, H. Ihara, Chem. Commun. (Camb.) 51 (2015) 14243-14246. DOI:10.1039/C5CC04966K |
| [4] |
A. Shen, Z. Guo, L. Yu, L. Cao, X. Liang, Chem. Commun. (Camb.) 47 (2011) 4550-4552. DOI:10.1039/c1cc10421g |
| [5] |
T. Liang, Q. Fu, A. Shen, et al., J. Chromatogr. A 1388 (2015) 110-118. DOI:10.1016/j.chroma.2015.02.019 |
| [6] |
A. Shen, X. Li, X. Dong, et al., J. Chromatogr. A 1314 (2013) 63-69. DOI:10.1016/j.chroma.2013.09.002 |
| [7] |
H. Zhang, X. Qiao, T. Cai, et al., Anal. Bioanal. Chem. 409 (2017) 2401-2410. DOI:10.1007/s00216-017-0187-z |
| [8] |
T. Cai, H. Zhang, A.F.M.M. Rahman, Y.P. Shi, H. Qiu, Microchim. Acta 184 (2017) 2629-2636. DOI:10.1007/s00604-017-2277-1 |
| [9] |
B. Yang, T. Cai, Z. Li, M. Guan, H. Qiu, Talanta 175 (2017) 256-263. DOI:10.1016/j.talanta.2017.07.038 |
| [10] |
H. Liu, J. Chen, Z. Li, et al., Talanta 164 (2017) 137-140. DOI:10.1016/j.talanta.2016.11.037 |
| [11] |
P. Jandera, P. Janás, Anal. Chim. Acta 967 (2017) 12-32. DOI:10.1016/j.aca.2017.01.060 |
| [12] |
S. Bocian, M. Skoczylas, B. Buszewski, J. Sep. Sci. 39 (2016) 83-92. DOI:10.1002/jssc.201500825 |
| [13] |
X. Zhang, S. Chen, Q. Han, M. Ding, J. Chromatogr. A 1307 (2013) 135-143. DOI:10.1016/j.chroma.2013.07.106 |
| [14] |
H. Liu, Y. Guo, X. Wang, et al., RSC Adv. 4 (2014) 17541-17548. DOI:10.1039/C4RA01408A |
| [15] |
T. Cai, H. Zhang, Z. Li, A.F.M.M. Rahman, H. Qiu, RSC Adv. 6 (2016) 32757-32760. DOI:10.1039/C6RA04824B |
| [16] |
Y. Li, L. Xu, T. Chen, et al., Anal. Chim. Acta 726 (2012) 102-108. DOI:10.1016/j.aca.2012.03.032 |
| [17] |
Y. Lin, X. Han, C.J. Campbell, et al., Adv. Funct. Mater. 25 (2015) 2920-2927. DOI:10.1002/adfm.201500321 |
| [18] |
Z. Li, Y. Liu, Y. Zhao, et al., Anal. Chem. 88 (2016) 10002-10010. DOI:10.1021/acs.analchem.6b02175 |
| [19] |
I. Shown, H.C. Hsu, Y.C. Chang, et al., Nano Lett. 14 (2014) 6097-6103. DOI:10.1021/nl503609v |
| [20] |
K. Zhang, M. Cao, C. Lou, et al., Anal. Chim. Acta 970 (2017) 73-81. DOI:10.1016/j.aca.2017.03.015 |
| [21] |
X. Liang, S. Liu, X. Song, Y. Zhu, S. Jiang, Analyst 137 (2012) 5237-5244. DOI:10.1039/c2an36091h |
| [22] |
Y. Li, L. Qi, H. Ma, Analyst 138 (2013) 5470-5478. DOI:10.1039/c3an01122d |
| [23] |
X. Liang, X. Wang, H. Ren, et al., J. Sep. Sci. 37 (2014) 1371-1379. DOI:10.1002/jssc.v37.12 |
| [24] |
H. Liu, Y. Guo, X. Wang, X. Liang, X. Liu, RSC Adv. 4 (2014) 37381-37388. DOI:10.1039/C4RA03432E |
| [25] |
Q. Wu, Y. Sun, X. Zhang, et al., J. Chromatogr. A 1492 (2017) 61-69. DOI:10.1016/j.chroma.2017.02.067 |
| [26] |
X. Liang, X. Hou, J.H.M. Chan, Y. Guo, E.F. Hilder, TrAC Trends Analyt. Chem. 98 (2018) 149-160. DOI:10.1016/j.trac.2017.11.008 |
| [27] |
A. Du, Z. Zhu, S.C. Smith, J. Am. Chem. Soc. 132 (2010) 2876-2877. DOI:10.1021/ja100156d |
| [28] |
P. Xu, J. Yang, K. Wang, Z. Zhou, P. Shen, Chin. Sci. Bull. 57 (2012) 2948-2955. DOI:10.1007/s11434-012-5121-3 |
| [29] |
T. Premkumar, K.E. Geckeler, Prog. Polym. Sci. 37 (2012) 515-529. DOI:10.1016/j.progpolymsci.2011.08.003 |
| [30] |
S. Han, D. Wu, S. Li, F. Zhang, X. Feng, Adv. Mater. 26 (2014) 849-864. DOI:10.1002/adma.v26.6 |
| [31] |
S. Blankenburg, M. Bieri, R. Fasel, et al., Small 6 (2010) 2266-2271. DOI:10.1002/smll.v6:20 |
| [32] |
Z. Li, X. Zhang, H. Tan, et al., Adv. Funct. Mater. (2018) 1805026. |
| [33] |
J. Yan, Z. Fan, W. Sun, et al., Adv. Funct. Mater. 22 (2012) 2632-2641. DOI:10.1002/adfm.201102839 |
| [34] |
Y. Guo, S. Gaiki, J. Chromatogr. A 1218 (2011) 5920-5938. DOI:10.1016/j.chroma.2011.06.052 |
| [35] |
R.I. Chirita, C. West, A.L. Finaru, C. Elfakir, J. Chromatogr. A 1217 (2010) 3091-3104. DOI:10.1016/j.chroma.2010.03.001 |
| [36] |
G. Jin, Z. Guo, F. Zhang, et al., Talanta 76 (2008) 522-527. DOI:10.1016/j.talanta.2008.03.042 |
| [37] |
M. Ramos Payán, B. M.Á.López, R. Fernández-Torres, M.V. Navarro, M.C. Mochón, J. Chromatogr. B 879 (2011) 197-204. DOI:10.1016/j.jchromb.2010.12.006 |
 2019, Vol. 30
2019, Vol. 30 

