Organic solar cells (OSCs), which featured with low-cost, largescale manufacturing and compatibility with flexible substrates, have attracted considerable attention in the past decades. In this field, the bulk heterojunction polymer solar cells (BHJ-PSCs) comprising a conjugated polymer as the donor material (D) and an acceptor material (A) have been used commonly in devices, which provide the enough D/A interface photogenerated electron-hole pairs, known as excitons, to separate into free charge carriers and create an electrical current upon collection at the electrodes [1]. With the continuous innovation in materials design, device engineering and working mechanism revealing, especially for the emergence of nonfullerene acceptors (NFAs), rapid progress of PSCs has been made in recent years [2, 3]. State-of-the-art power conversion efficiencies (PCEs) have been exceeding 14% [4-7], showing their great potentials for future practical applications.
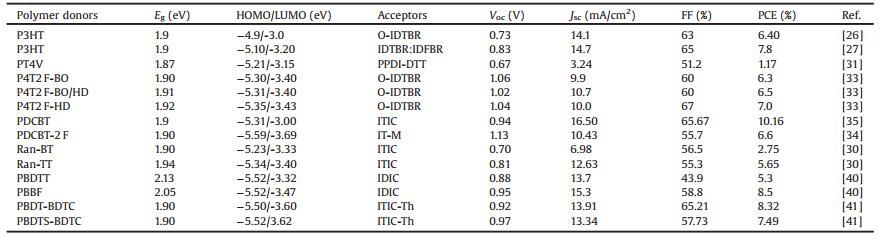
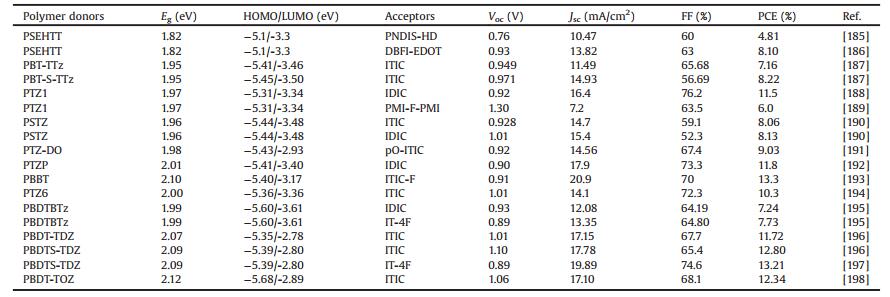
As is well known to us, the commonly accepted working mechanism of BHJ OSCs involves four fundamental steps: (1) light absorption and exciton generation, (2) exciton diffusion to the D/A interface, (3) exciton dissociation at the D/A interface, (4) charge transport and collection [8]. On the other hand, photocurrent generation in BHJ OSCs can occur via (ⅰ) channel Ⅰ: donor excitation followed by electron transfer to the acceptor and (ⅱ) channel Ⅱ:acceptor excitation followed by hole transfer to the donor (Fig. 1a) [9]. Channel Ⅰ is the predominant mechanism in fullerene based OSCs, due to the low absorption ability of fullerene derivatives. In contrast, in NFA based PSCs (NF-PSCs), both mechanisms play key roles in photocurrent generation because of the strong absorption of both donor and acceptor materials. Therefore, in developing highly efficient OSCs, synthesis of donor and acceptor semiconductors with sufficiently high and complementary absorption coverage of the solar spectrum is of great importance. On the basis of their optical bandgaps (Egopts), organic semiconductors can be classified as low bandgap (LBG, Egopt < 1.6 eV), medium bandgap (MBG, 1.6 eV < Egopt < 1.8 eV), and wide bandgap (WBG, Egopt > 1.8 eV) (Fig. 1b) [10]. In the early stage, fullerene derivatives dominated the acceptors in PSC filed, LBG and MBG polymer donors have been extensively studied, and high PCE of 11.48% have been certified [11]. In contrast, owing to the limited light absorption in visible light region and relatively low photocurrent output, the performance of WBG polymers lacked behind yet. Only few successful WBG polymer based single-junction binary devices showed high PCEs over 10% [12-16]. In despite of that, WBG polymers still play key role in OSC filed, and have been widely used in the tandem solar cells and ternary blend solar cells [10]. Nevertheless, owing to the inherent drawbacks of fullerenes, the growing research attention turned to focus on developing OSCs based on the more promising NFAs [2, 3]. The NFAs have shown great potential to replace fullerene derivatives due to their easily tunable chemical structures and energy levels, improved light absorption in visible region, enhanced photostability and morphological stability [2, 17]. What is more, the recently great progress of NFAs, especially the highly efficient near-infrared NFAs enabled the WBG polymers to break through the light harvesting limitations and create new development opportunities [2, 18]. In addition, WBG polymers with deep highest molecular orbital levels (HOMOs) matched with appropriate near-infrared NFAs could also generate extremely high open-circuit voltage (Voc) with reduced energy loss (Eloss, defined as Eloss = Egopt – qVoc, where the Egopt is the smallest optical bandgap of the used donor and acceptor materials, Voc is the open-circuit voltage of the device, and q is the elemental charge). Up to date, the most efficient NF-OSCs are based on WBG polymer donors [4-6], indicating their great potentials in NF-OSC field.
|
Download:
|
| Fig. 1. (a) Schematic illustration of the channel Ⅰ and Ⅱ excitations in OSCs. (b) Solar spectrum and absorption spectra of different bandgap polymers. | |
Herein, we present the review of recent developments in WBG donor polymers and their applications in OSCs to help understand the structure-property relationships of WBG polymers. A systematic introduction and summary of the development of WBG polymers and their versatile applications in OSCs, especially in fullerene based PSCs, have been provided by Sun et al. [10]. Consequently, this review focuses on the recent progress of WBG polymers applied in NF-PSCs. The early stage of WBG polymer development and their applications in fullerene-based OSCs is beyond the scope of this review.
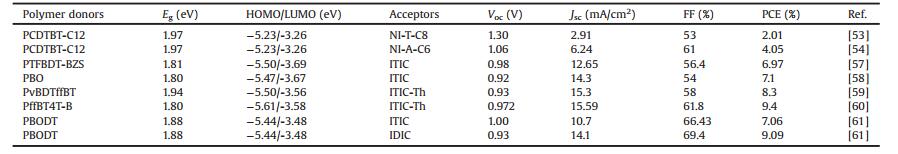
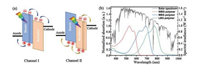
2. D-type donor polymers 2.1. Polythiophene-based homopolymersPolythiophene (PT) and its derivatives have attracted consider- able attention in the past decades due to their versatile applications promising applications in many fields [19]. Among them, poly(3-hexylthiophene) (P3HT, Eg =1.9 eV) is the most representative conjugated polymer donor, with the advantages of higher hole mobility and crystalline structure, which makes it easy to form a nanoscale interpenetrating network with fullerene derivative acceptors [20]. The simple chemical structure and straightforward synthetic pathway of P3HT make it as one of the most suitable candidates for large scale manufacturing of PSCs [21]. However, P3HT has a relatively high HOMO level (-4.9 eV), while PCBM has a lowlowest unoccupied molecularorbital level (LUMO ≈-3.91 eV) [22]. The small energy offset between P3HT and PCBM limited the open circuit voltage (Voc) value ~0.6 V) and the PCE (~4%). Using high LUMO acceptors, such as ICBA (-3.74 eV) and IC70BA (-3.72 eV) could improve the Voc up to 0.87 V and PCE up to 7.4% [22, 23]. Moreover, fine-tuning the energy level by using NFAs, the Voc could be further improved over 1.20 V [24, 25], showing the superiorities of tunable NFAs in the energy level alignment. Using LBG NFAs with strong absorption in longer wavelength, the short current density (Jsc) could be significantly improved, giving rise to the high PCE of 6.4% in P3HT:IDTBR and 7.8% in P3HT:IDTBR:IDFBR devices [26, 27]. On the other hand, the overall properties of PT derivatives have also been modulated by chemical modifications, such as introducing various side chains [19, 28-31], cyano groups [32] and fluorine atoms [33, 34]. Zhang et al. attached the PT backbone with electron-withdrawing carboxylate side chains and developed a new PT based WBG polymer PDCBT (Eg =1.9 eV) [29]. PDCBT had a lower HOMO level (-5.24eV vs. -4.9 eV) than P3HT, thus improved Voc up to 0.9 V in PDCBT:PC71BM devices. The higher absorption and better crystallinity also enabled a high PCE of 7.2% in the resulting devices. Blending PDCBT with ITIC, a much higher PCE of 10.16% could be obtained [35]. In contrast, P3HT:ITIC devices only achieved an extremely low PCE of 1.25%. By introducing fluorine atoms onto above PDCBT main chains, a further lower-lying HOMO level of -5.59 eV was realized in the resulting PDCBT-2 F (Eg =1.9 eV), giving rise to significantly improved Voc of 1.13 eV and suppressed Eloss of 0.46 eV [34]. The chemical structures of PT based donor homopolymers are presented in Fig. 2, and the corresponding photovoltaic parameters of NF-PSCs are summarized in Table 1.

|
Download:
|
| Fig. 2. Chemical structures of PT and BDT based WGB homopolymers. | |
|
|
Table 1 Polymer characteristics and the corresponding device performance of NF-OSCs. |
2.2. Benzodithiophene-based homopolymers
In 2008, benzo[1, 2-b:4, 5-b']dithiophene (BDT) unit was first used in synthesis of photovoltaic polymers by Hou et al. [36]. Since then, hundreds of BDT based semiconductors have been developed. BDT unit features a weak electron-donating, rigid and large coplanar structure, which has become one of the most successful building blocks in synthesis of highly efficient photovoltaic materials [37]. The rigid and planar conjugated structure of BDT makes it attractive for achieving highly tunable energy levels and optical band gaps as well as high hole mobilities [37]. The BDT homopolymers have a typical wide bandgap with the Egopt around 2.0 eV. Modifying the BDT with alkylthienyl or alkylthiothienyl side chains, a deeper HOMO below -5.4 eV could be realized, giving rise to high Voc over 0.9 V and PCE over 6.0% when blending with PC71BM [38, 39]. If fluorinated the alkylthienyl side chains, the HOMO level of PBBF (Eg = 2.05 eV) was further lowered to -5.52 eV, generating a high Voc of 0.95 V and PCE of 8.5% in PBBF:IDIC based NF-OSCs without extra treatments [40]. If substituted the alkylthienyl side chains with electron-withdrawing alkylcarboxyl side chains, the HOMO level of PBDT-BDTC (Eg =1.90 eV) could also be lowered to -5.50 eV [41]. The PBDT-BDTC:ITIC-Th devices generated a high Voc of 0.97 V and a PCE of 7.30%, which was further improved to 8.32% by optimizing the morphology with adding 0.5% (by volume ratio) of 1, 8-diiodooctane (DIO). The chemical structures of BDT based homopolymers are presented in Fig. 2, and the corresponding NF-PSC photovoltaic parameters are summarized in Table 1.
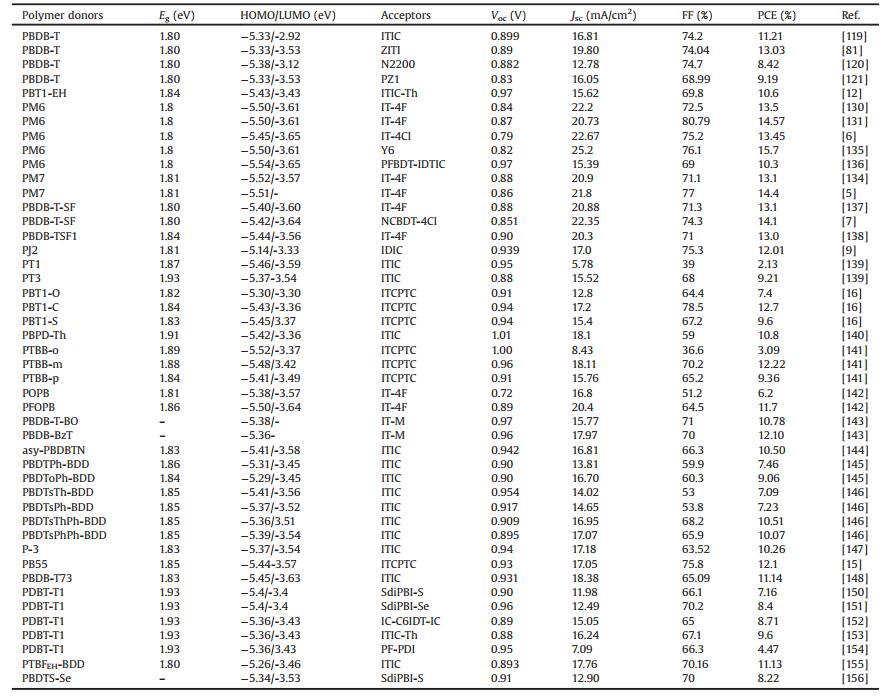
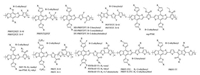
2.3. D1-D2 type donor polymersThe D1-D2-type copolymer here is classified as a donor polymer in which the molecular backbone consists of the alternating electron-rich units [10]. Many reports also classified some of these copolymers as the D—A type, due to partial electron-rich units in which modified with electron-withdrawing substituents could act as "weak electron acceptors" [42, 43]. Among the D1-D2-type copolymers, BDT and thiophene alternated copolymers are the most representative ones [44-49]. Firdas et al. reported a series of BDT and thiophene alternated copolymers, PBDT[2 H]T (Eg =2.1 eV), PBDT[2 F]T (Eg =2.1 eV) and PBDT(T)[2 F]T (Eg =2.0 eV), by introducing fluorine atom on the thiophene backbone and modifying the BDT with different side chains [47]. The fluorination and alkylthienyl group substitution lowered the energy levels and reduced the charge recombination losses in the related devices. For these reasons, PBDT(T)[2 F]T:ITIC devices obtained the significantly improved PCE of 9.8%. Xia et al. combined fluorinated bithiophene (2 FT) with the BDT derivatives that had different side chain lengths varying from 2-hexyldecyl (HD) to longer 2- octyldodecyl (OD) and 2-decyltetradecyl (DT), and developed three PBDT2 FT-based polymers of HD-PBDT2 FT, OD-PBDT2 FT and DT-PBDT2 FT [50]. They found that the internal quantum efficiency (IQE) of the PBDT2 FT:PC71BM devices decreased dramatically with increasing length of the donor side chain, while the quantum efficiency of the PBDT2 FT:ITIC solar cells was independent on the side chain length. Their in-depth studies suggested that the mechanism for morphology evolution was different in the nonfullerene system from fullerene system, possibly due to the lack of structural symmetry of ITIC compared to PC71BM. Kong et al. copolymerized 2 FT with alkylthio-substituted thieno[3, 2-b]thiophene unit (STT) and developed a donor copolymer of PSTTF2T (Eg =1.89 eV) [51]. Compared with the alkoxy-substituted analogue POTTF2T, the sulfur atom introduction significantly lowered the HOMO level from -5.18 eV to -5.36 eV, and improved the Voc from 0.60 V to 0.93 V when blended with ITIC. The more suitable phase separation also contributed to the better charge separation and higher PCE of 5.22% in PSTTF2T:ITIC devices. On the other hand, modification of thiophene backbone with alkylcarboxyl groups could further lower the HOMO level below -5.4 eV [48, 49]. 3MT-Th with a methylcarboxyl substituted on thiophene backbone exhibited a large Eg of 2.02 eV [48]. The resulting 3MT-Th:ITIC devices could perform well in halogen-free solvents, such as toluene and o-xylene. The best efficiency of 9.73% was realized finally. Liu et al. modified the thiophene backbone with methylcarboxyl groups and developed two WBG polymers of ran-PThE (Eg =1.99 eV) and reg-PThE (Eg =1.99 eV), using random or regioregular linkage respectively [49]. The regioregular polymer reg-PThE exhibited higher order packing and absorption than the random ran-PThE and generated a higher PCE of 10.57% using ITIC as the acceptor material. If replacing ITIC with FTIC, the crystallinity of reg-PThE:FTIC was improved and the PCE was further elevated to 12.07%. Hou et al. reported the synthesis of two WBG polymers of PB2T (Eg =2.24 eV) and PB3T (Eg =1.96 eV) based on BDT and alkylcarboxyl substituted oligothiophene units [52]. The PB3T with a thiophene bridge between the two alkylcarboxyl substituted thiophene units could lower the steric hindrance significantly, leading to a higher crystallinity. The PB3T:IT-M devices dealing with chlorobenzene (CB) obtained a much higher PCE of 11.7%, whereas seriously poor performance of 0.01% was achieved in PB2T:IT-M devices. What is more, the PB3T:IT-M could also exhibit a slightly better PCE of 11.9% using a green solvent of anisole. In their following studies, a series of copolymers of PDTB-EF-T1, PDTB-EF-T2, PDTB-EF-T3 were reported, in which fluorine atom was further introduced and the alkylcarboxyl-based side chains were fine tuned [4]. The continuous fluorination lowered the HOMOs below-5.50eV, making them obtain high Voc even blended with IT-4F acceptor. Because the linearly decyl-substituted PDTB-EF-T2 exhibited stronger interchain π-π interaction and more ordered π-π stacking, it showed a higher hole mobility compared to the counterparts. For this reason, the most symmetric charge transport and suppressed charge recombination were achieved in the related blend film, which contributed to the higher Jsc and improved FF in PDTB-EF-T2:IT-4F devices, giving rise to the extremely high PCE of 14.2%. By reducing the number of alkylcarboxyl substituted thiophene units, the other two WBG polymers of PBDT-2TC (Eg =1.96eV) and PBDT-S-2TC (Eg =1.94eV) were reported by An et al. [42]. Removing one carboxylate from the backbone could reduce the repulsion dramatically between the adjacent units and favor a tight π-π stacking, compared to PB2T. A reasonable HOMO level offset in relation to that of ITIC acceptor and balanced hole and electron transport were observed for both donor copolymers. As a result, the PSCs based on PBDT-S-2TC:ITIC exhibited a high PCE of 10.12% with a high Voc of 0.96V. When replacing alkylcarboxyl substituted thiophene group with alkylcarboxyl substituted TT unit, the crystallinity of PBDT-TT (Eg =1.88eV) could be further improved [43]. Thus, high PCEs of 9.67%, 11.38% and 11.03% were obtained in NF-PSCs based on ITIC, IT—M and 6TIC, respectively. The chemical structures of D1-D2 type donor copolymers are presented in Fig. 3, and the corresponding NF-PSC photovoltaic parameters are summarized in Table 2.
|
Download:
|
| Fig. 3. Chemical structures of D1-D2 type WBG copolymers. | |
|
|
Table 2 Polymer characteristics and the corresponding device performance of NF-OSCs. |
3. D-A type donor copolymers 3.1. Benzothiadiazole-based copolymers
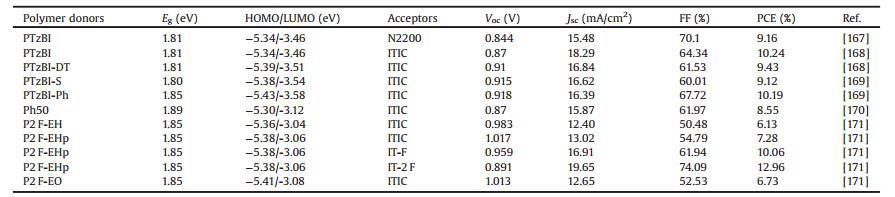
2, 1, 3-Benzothiadiazole (BT) has been widely used for constructing D—A LBG and MBG copolymers, due to its strong electron-accepting ability. Using fullerene derivatives as the acceptors, high efficiencies up to 11.7% have been realized in the MBG polymer-based PSCs processed from hydrocarbon solvents [11]. Our group also improved the PCE over 10% of BT-based WBG copolymers through rational material design [14]. In 2015, another new WBG polymer of PCDTBT-C12 (Eg=1.97eV) was developed by Bo et al., which was applied to fabricate NF-PSCs using a WBG acceptor of NI—T—NI (Eg =2.54eV) [53]. Owing to the limited absorption range, a relatively low PCE of 2.01% was obtained. However, an unprecedented high Voc of 1.30V was successfully realized in such devices. By replacing the thiophene bridge of NI—T—NI with alkynyl bridge and attaching 1, 8-naphthalimide with different length alkyl chains to fine tune the properties of acceptors, they further improved the PCE of PCDTBT-C12:NI-A-C6 devices to 4.05% even in a thin film thickness of about 30 nm [54]. BDT-BT containing copolymers are typical highly efficient MBG polymers for NF-PSCs [55, 56]. Attaching alkylphenyl side chains on the BDT units and alkoxy chains on the BT units could enlarge the Eg over 1.80 eV [57]. The WBG copolymer of PTFBDT-BZS obtained a moderate PCE of 6.97% when blended with ITIC, which was further improved to 9.25% in PTFBDT-BZS:ITIC:PC71BM devices [57]. Using 2, 1, 3-benzoxadiazole (BO) instead of BT, the PCE was slightly improved to 7.1% in PBO:ITIC devices [58]. By vertical connecting the BDT group, Yan et al. developed a new WGB copolymer of PvBDTffBT (Eg =1.94 eV), which produced a much higher PCE of 8.3% than the traditional BDT-based PBDTffBT (PCE = 3.27%) [59]. The more twisted backbone of PvBDTffBT reduced its aggregation tendency, leading to better phase separation. On the other hand, they inserted a benzene ring in the backbone of PffBT4T and developed another WBG copolymer of PffBT4T-B (Eg = 1.80 eV), and the PCE was improved from 6.6% (PffBT4T) to 9.4% (PffBT4T-B) when using ITIC-Th as the acceptor material [60]. The strong interdigitation of PffBT4T-B chains expelled ITIC-Th from the polymer domains, contributing to more pure and crystalline domains of ITIC-Th, and thus leading to a higher electron mobility and FF value. Chen et al. synthesized a dithienobenzoxadiazole (DTBO)-based WBG copolymer of PBOT (Eg =1.88 eV) [61]. Without any additive and post-treatment, the PSCs based on PBODTand ITIC showed a good PCE of 7.06%. When replacing ITIC with IDIC, an improved PCE of up to 9.09% was obtained with a slightly larger energy loss of 0.69 V. Furthermore, the as-cast devices processed from single nonchlorinated solvent of 1, 2, 4-trimethylbenzene (TMB) could achieve a good PCE above 8%. The chemical structures of BT-based copolymers are presented in Fig. 4, and the corresponding NF-PSC photovoltaic parameters are summarized in Table 3.

|
Download:
|
| Fig. 4. Chemical structures of BT and its derivatives based WBG copolymers. | |
|
|
Table 3 Polymer characteristics and the corresponding device performance of NF-OSCs. |
3.2. Benzotriazole-based polymers
Compared with BT unit, benzotriazole (BTA) has a weaker electron-deficient strength, where the lone-pair electrons on the 2-position of N can donate onto the triazole ring, leading to the higher LUMO and larger bandgaps in the related copolymers [62, 63]. BTA-based WBG copolymers have been extensively studied in the past few years (Fig. 4). Li et al. developed a series of BTA-based WBG copolymers named from J40 to J91 by varying different side chains [64-80]. Matched with appropriate NFA acceptors, the PCE has exceeded over 13% [81]. Apart from the common side chain engineering, Huang et al. modified the side chain of BTA with alkyl-siloxane (SiO) to manipulate the molecular stacking and orientation [82]. The resulting copolymer PBTA-Si (Eg = 1.93 eV) was then used to fabricate efficient ternary blend and all-polymer solar cells (all-PSCs) with a thick active layer. The optimized all-PSCs achieved a PCE of 9.17% with an active layer thickness of 350 nm, which could maintain 8% even with the thicknesses over 400 nm. If modification of BTA with polyethylene glycol (PEG) side chains, copolymer PBTA-PEG-2% (Eg = 1.97 eV) could produce a high PCE of 9.27% in ternary blend devices processed with the friendly solvent of 2-methyl-tetrahydrofuran (MeTHF). Yang et al. developed a series of BTA-based copolymers containing asymmetric BDT building blocks [83-85], which could also realize a high PCE up to 11.56% when blended with ITIC [84]. The result demonstrated the application potential of asymmetric side chain engineering. By vertical connecting BDT unit in polymer main chain, Yan et al. developed a new WGB copolymer of PvBDTTAZ (Eg = 2.05 eV) [86]. PvBDTTAZ-based devices could achieve a high PCE = 11.6% while maintaining a high Voc of 1.08 V and a low Eloss of 0.55 V. The high device performance was enabled by favorable morphology of the blend films, in which both the donor polymer and NFA could maintain high crystallinity and form small domains at the same time. Most recently, our group developed a new donor block, dithienothiapyran (DTTP), which had more planar backbone than the dithienopyran (DTP) analogue. The resulting copolymer PDTTP-FBTA showed more linear backbone, higher crystallinity and lower HOMO level. PDTTPFBTA:IT-M-based devices obtained a high PCE of 10.51%, which was further improved to 11.57% by adding a small amount of a WBG acceptor of meta-TrBRCN due to the improved absorption and optimized morphology. To further improve the crystallinity of BTA-based polymers, naphtho[1, 2-c:5, 6-c]bis(2-octyl-[1-3]triazole) (TZNT) was also used, which could be considered as two BTA units fused as an angular shape [62, 87]. Adopting fluorinated-bithiophene (DTF) as the donor block, PDTF-TZNT exhibited higher crystallinity and stronger absorption ability than the nonfluorinated counterpart (PDTH-TZNT), giving rise to a higher PCE of 10.60% and 11.48% in binary and ternary blend PSCs. Because of the relatively high HOMO level of PDTF-TZNT (-5.24 eV), the PCE was still limited by the poor Voc (0.8 V). This could be resolved by modifying BDT block with sulfuration (BDTS) or both sulfuration and fluorination (BDTSF) at the same time [87]. PBDTSF-TZNT:IT- 4 F devices obtained a high PCE of 13.25% with a low Eloss of 0.59 eV. Homo-tandem devices were also fabricated to promote the PCE up to 14.52%, which is the best record for organic homo-tandem cells at present. The chemical structures of BTA-based copolymers are presented in Fig. 5, and the corresponding NF-PSC photovoltaic parameters are summarized in Table 4.

|
Download:
|
| Fig. 5. Chemical structures of BTA and its derivatives based WBG copolymers. | |
|
|
Table 4 Polymer characteristics and the corresponding device performance of NF-OSCs. |
3.3. Benzodithiophenedione-based copolymers
Benzo[1, 2-c:4, 5-c']dithiophene-4, 8-dione (BDD) has a planar rigid backbone and the two C=O bonds endow BDD block with electron-accepting property. In 2012, Hou et al. reported a BDDbased WBG copolymer, PBDB-T (or namely PBDTBDD, Eg = 1.80 eV), which generated a high PCE of 6.67% in PBDB-T:PC71BM devices and a moderate PCE of 4.39% in PBDB-T:SDIPBI devices [117, 118]. By matching PBDB-T with the LBG acceptor of ITIC, a PCE over 11% was achieved by the same group [119]. After that, PBDB-T has been widely applied to fabricate highly efficient NF-PSCs, and the PCEs have been promoted over 13% [81]. If introducing sulfur atom on the two dimensional (2D) side chains [122], fluorine [123] or chlorine [124] on the 4-position of thiophene bridges, and using fluorinated bithiophene as the bridges [125], the resulting polymers (PBDB-TS, PFBDB-T and PCl(4)BDB-T, PBDB-BT) exhibited lower bandgaps around 1.78 eV. Nevertheless, using longer alkyl side chains, the related copolymers of PBT1-MP (Eg = 1.86 eV), PBT1-EH (Eg = 1.84 eV), and PBT1-BO (Eg = 1.81 eV), could still maintain the wide bandgaps [12]. Especially for introduction of chlorine atom on the 3-position of thiophene bridge, a larger bandgap of 2.11 eV was achieved in PCl(3)BDB-T, due to the more twisted polymer main chain [124]. If introducing chlorine atom on the 4-position of thiophene bridge and replacing the alkylthienyl side chains with alkylphenyl chains, PBT1-C-2Cl showed improved absorption, lowered energy levels and higher crystallinity than PBT1-C [126]. The higher charge carrier mobilities, enhanced phase separation and reduced charge recombination elevated the PCE from 10.9% (PBT1-C:IT-4 F) to 12.7% (PBT1-C-2Cl:IT-4 F). On the other hand, fluorination or chlorination of 2D alkylthienyl side chains have been proven to be an effective strategy to lower the HOMO level, improve the absorption and crystallinity of the resulting copolymers, such as PM6 (or namely PBDB-T-2 F) and PM7 (or namely PBDB-T-2Cl) [5, 6, 127-134]. Thus, high PCEs over 14% have been realized in both the binary and ternary blend devices [5, 6, 131, 132]. Most recently, Zou et al. matched PM6 with a new designed NFA, and realized an unprecedented high PCE of 15.7%, which is a new record for single-junction solar cells [135]. Yan et al. blended PM6 with a LBG polymer acceptor, and achieved a high PCE of 10.3%, which is the best efficiency for all-PSCs based on WBG donor polymers [136]. Combining the sulfuration and fluorination on the 2D alkylthienyl side chains could further lower the HOMO level of the resulting polymers of PBDB-T-SF (Eg = 1.80 eV) and PBDB-T-SF1 (Eg = 1.84 eV) [7, 137, 138]. Selecting appropriate NFAs, high PCE of 14.1% and low Eloss of 0.55 eV could also be realized [7]. In contrast, removing the thiophene bridge would result in a more twisted polymer main chain of PT1 (Eg = 1.87 eV), which induced poor blend morphology [139]. If replacing thiophene bridge with thienothiophene (TT) bridge, PT3 (Eg = 1.83 eV) could adopt a zigzag backbone conformation, which was then afforded with feasible solution processability and increased aggregation to deliver higher PCE of 9.21% even without extra treatment [139]. Yang et al. used the alkylsilylthienyl side chains instead of alkylthienyl side chains and developed a WBG copolymer of PJ2 (Eg = 1.81 eV) [9]. They observed the wellintermixed blend morphology containing enhanced small molecular acceptor (SMA) order ranges with mixed face-on and edge-on orientations. Such an optimal microstructure generated a high efficiency of 12.01% in PJ2:IDIC devices. Using the functionalized phenyl groups instead of the 2D alkylthienyl side chains, the resulting copolymer exhibited slightly enlarged bandgap and lower-lying HOMO level [16, 140-142]. It was found that the appropriate type (alkoxy, alkyl, alkylthio) [16] and the suitable position (ortho, meta, para) [141] of the side chains had a significant effect on the polymer packing behavior and the blend morphology. Among which, alkyl chains on the para-position (PBT1-C) or alkylthio chains on the meta-position (PTBB-m) of phenyl groups would be favorable for more ideal phase separation to induce better device performance [16, 141].
Developing NF-PSCs with green solvent processability is meaningful for the practical applications. Due to the limited solubility of the materials, the scope of solvent selection is relatively narrow. Therefore, the aromatic solvents still need for fabricating NF-OSCs. Hou et al. introduced the extended π-conjugated benzothiophene attached with long alkyl group as side chains to guarantee the solubility of PBDB-T-BzT, which exhibited good solubility even in tetrahydrofuran (THF) [143]. PBDB-T-BzT: IT-M device processed from THF exhibited a high PCE of 12.10%, which was better than PBDB-T:IT-M (11.74%) device processed from chlorobenzene (CB). Yang et al. reported a series of asymmetric benzodithiophene-based WBG copolymers (asy-PBDB-TN, PBDToPhPh-BDD, PBDTPh-BDD, PBDTsTh-BDD, PBDTsPh-BDD, PBDToPh-BB, PBDTsThTh-BDD and PBDTsPhPh-BDD), highlighting the important role of 2D side chain on morphology control for NF-PSCs [144-146].
Random terpolymer strategy is a promising design method for further improving the advantageous properties of copolymers. Terpolymers exhibit several desirable advantages, such as high solubility, broad absorption range, finely tuned energy levels, and phase separation in the blend films [147]. By side chain engineering [15] and the backbone modification [147, 148], the overall properties of random terpolymers could be fine-tuned to maximize the device performances. For those reasons, random terpolymers based on BDD unit (PB55) could also achieved a high PCE over 12% [15].
Enlarging π-conjugated system with fused aromatic rings has been demonstrated to be a useful strategy to improve the planar molecular conformation, leading to the enhanced charge carrier transport. Sun et al. fused two thiophenes on the BDT skeleton and synthesized a new building block of dithieno[2, 3-d; 2', 3'-d']benzo [1, 2-b; 4, 5-b']dithiophene (DTBDT) [149]. PDBT-T1 (Eg =1.85 eV) had a highly rigid backbone and formed well defined fibril-like morphology in PDBT-T1:PC71BM blend, giving rise to a high PCE of 9.7%. When blending PDBT-T1 with PDI-based WBG NFAs [150, 151], a high PCE of 8.4% could be achieved with a large FF value up to 70% [151]. In 2016, Zhan and Sun et al. matched PDBT-T1 with fused-ring acceptor materials, yielding a high PCE of 8.7% [152], which was further improved more than 9% later [153]. Afterwards, PDBT-T1 was also applied to fabricate all-PSCs, which showed a PCE of 4.47% [154]. In addition, BDT analogue (thieno[2, 3-f] benzofuran (TBF)) [155] and BDD derivative (1, 3-di(thiophen-2-yl)-selenopheno[3', 4':4, 5]benzo[1, 2-c]thiophene-4, 8-dione) [156] have also been used to construct WBG copolymers. PTBFEH-BDD and PBDTS-Se exhibited good photovoltaic properties as well. The chemical structures of BDD-based copolymers are presented in Fig. 6, and the corresponding NF-PSC photovoltaic parameters are summarized in Table 5.

|
Download:
|
| Fig. 6. Chemical structures of BDD and its derivatives based WBG copolymers. | |
|
|
Table 5 Polymer characteristics and the corresponding device performance of NF-OSCs. |
3.4. Thienopyrroledione-based copolymers
Thieno[3, 4-c]pyrrole-4, 6-dione (TPD) is a versatile unit, which possesses relatively simple, compact, symmetric and planar structure. These properties make it be beneficial for electron delocalization, which promote intrachain and interchain interactions when incorporated into various conjugated copolymers. The relatively strong electron-withdrawing effect can lead to lowlying HOMO level, which is desired to increase Voc in PSCs. BDT-alt-TPD-based copolymers can also be used in all-PSCs [157-166]. Ma et al. reported a series of BDT-alt-TPD-based copolymers of P8, PT8 and PTP8 by side chain engineering on both BDT and TPD units [157]. This type of copolymers had low-lying HOMOs below -5.50 eV, giving rise to high Voc up to 1.0 V when using N2200 as the acceptor material [157]. If using P(NDI2HD-T) or P(NDI2HD-T2 F) instead of N2200, both PT8 and PTP8 devices showed higher PCEs over 6% [158, 159, 162]. Wang et al. reported two WBG copolymers of PBDT-TPD and PBDTS-TPD, by attaching a branched alkyl side chain on TPD skeleton or introducing sulfur atom on BDT side chain [163]. The HOMO levels of the resulting copolymers were lowered to -6.10eV and high Voc of 1.10V was obtained in PNDI-T-based devices. The PBDTS-TPD:PNDI-T devices showed a high PCE of 8.0%, which was higher than the PC71BM counterpart (PCE=7.1%). In contrast, if using a small molecular NFA of ITIC, only a moderate PCE of 5.4% was achieved in PT8 devices [167]. By introducing two thiophene bridges into the polymer main chains, the crystallinity of PBDT(T)-HT-TPD was improved, giving rise to the significantly improved PCE of 10.2% in ITIC-based devices [168]. On the other side, all-PSCs based on PBDT(T)-HT-TPD:P(NDI2HD-Se) could also be achieved a good PCE of 6.8% [164]. If attaching the alkyl groups on thiophene unit that facing the BDT skeleton, the symmetrical arrangement of the alkyl side chains would be broken, leading to the poor crystallinity [168]. For this reason, low PCE (~6%) were observed [9]. To deepen the HOMO energy level and enhance intermolecular interactions of TPD-based copolymers, Liang et al. introduced 4-methoxy thiophene (MOT) as conjugated side chains on BDT [169]. As expected, the MOT-substituted PMOT16 exhibited higher crystallinity than the control polymer of PBDTT-6ttTPD, leading to the significantly improved Jsc and FF in devices. Among which, PMOT16:IDIC devices exhibited a high PCE of 10.04%. A longer alkyl chains onTT bridge and asymmetric side chains on the BDT unit (PMOT40) could further improve the PCE up to 12.2% [170]. By increasing the fluorine atoms on the side chains, Jin et al. developed a series of TPD-based copolymers of P1, P2 and P3 [165]. Increasing the number of fluorine atom could lower the HOMO level gradually without changing the bandgap but improved the light absorption capabilityand carrier transport property, resulting in high Voc and Jsc values in all-PSCs. The stronger face-on orientation, tighter π-π packing, and the optimal blend morphology made P2:P(NDI2HD-Se)-based all-PSCs exhibit enhanced charge mobility and better PCE of 7.13%. Zhu et al. designed a WBG copolymer of PBDTNS-TPD based on alkylthionaphthyl-substituted BDT unit, which showed the better light-harvesting and chargetransport properties than PBDTBS-TPD[171]. Therefore, PBDTNS-TPD:ITIC devices showed a higher PCE of 8.19% than PBDTBS-TPD:ITIC devices (PCE=5.93%). Indacenodithiophene (IDT) block and random terpolymer strategy have also been employed to construct the TPD-based WBG copolymers for NFPSCs [166, 172]. The chemical structures of TPD-based copolymers are presented in Fig. 7, and the corresponding NF-PSC photovoltaic parameters are summarized in Table 6.
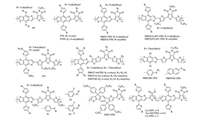
|
Download:
|
| Fig. 7. Chemical structures of TPD based WBG copolymers. | |
|
|
Table 6 Polymer characteristics and the corresponding device performance of NF-OSCs. |
3.5. Pyrrolobenzotriazoledione-based copolymers
In 2016, Huang et al. developed a new electron-deficient block of pyrrolo[3, 4-f]benzotriazole-5, 7-dione (TzBI), which could be considered as cyclic-imide functionalized BTA [173]. By replacing two hydrogen atoms on BTA unit with cyclic-imide, both the HOMO and LUMO energy levels of the resulting copolymers could be decreased, which was beneficial to increase Voc and PCE of the related PSCs. Furthermore, the accessibility of the N atom on the imide cycle provides a straightforward route for alkylation to promote the solubility of the resulting copolymers [173]. For these reasons, all-PSCs based on PTzBI:N2200 processed with a green solvent of 2-methyl-tetrahydrofuran (MeTHF) exhibited a more favorable film morphology than those processed with commonly used halogenated solvents, such as 1, 2-dichlorobenzene and chloroform, which presented a remarkable PCE of 9.16% [174]. Using ITIC as the acceptor, the PCE was further improved to 10.24% [175]. However, PTzBI-DT (Eg = 1.81 eV) with extended π-conjugation obtained poor phase separation in the PTzBI-DT:ITIC blend, leading to the reduced device performance (9.43%) [175]. Huang et al. modified the BDT units with alkythiothienyl and alkylphenyl groups, respectively, and reported the copolymers of PTzBI-S (Eg = 1.80 eV) and PTzBI-Ph (Eg =1.85 eV) with further lower-lying HOMO levels [176]. PTzBI-Ph exhibited enhanced hole mobility and higher crystallinity than PTzBI-S, giving rise to a higher PCE of 10.19%. They combined the alkylthienyl and/or alkylphenyl substituted BDT units with the alkoxy-substituted TzBI block (TzBI-O) and synthesized a series of random terpolymers (Ph00, Ph25, Ph50, Ph75, Ph100) [177]. These terpolymers exhibited gradually lowered HOMO energy levels and gradually increased Vocs in devices. The Ph50-based NF-PSC devices exhibited the highest PCE of 8.55%. They further introduced fluorine atoms on the alkylphenyl side chains and optimized the branching point, and developed three WBG copolymers of P2 F—EH, P2 F-EHp and P2 F—EO, which had the same Egs of 1.85 eV [178]. With the branching point moving away from the polymer backbone, the HOMO and LUMO levels would be lowered but the aggregation would be enhanced. In the meantime, they studied the number effect of fluorine atoms on the final device performance. The P2 F-EHp:IT-2 F devices exhibited a decent PCE of about 13% based on a device area of 0.05 cm2. This efficiency could maintain at 12.25% even for a 1 cm2 large-area device. The chemical structures of TzBI-based copolymers are presented in Fig. 8, and the corresponding NF-PSC photovoltaic parameters are summarized in Table 7.
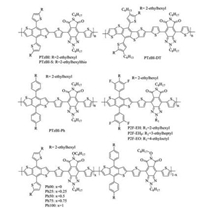
|
Download:
|
| Fig. 8. Chemical structures of TzBI based WBG copolymers. | |
|
|
Table 7 Polymer characteristics and the corresponding device performance of NF-OSCs. |
3.6. Quinoxaline-based copolymers
Quinoxaline (Qx) is a type of strongly electron-deficient skeleton, which has been widely employed as the acceptor building block to construct efficient D—A conjugated copolymers, especially for the MBG copolymers. A few successful cases with wide bandgaps have also been reported and applied in NF-PSCs. In 2016, Zou et al. replaced the alkylthienyl side chains of MBG copolymer PffQx-T (Eg =1.73 eV) with alkylthiophenyl side chains, and developed a new WBG copolymer PffQx-PS (Eg = 1.81 eV) with a lower-lying HOMO level [179]. The resulting devices using ITIC acceptor exhibited largely improved Voc from 0.89 V to 0.97 V, giving rise to the high PCE of 9.12%. Li et al. designed and synthesized a simple Qx-based donor copolymer of PTQ10 (Eg =1.92 eV) with a simple molecular structure [180]. PTQ10 had a low-cost synthetic route with a high overall yield of 87.4% via only two-step reactions from cheap raw materials. The optimized PSCs with PTQ10 as donor and IDIC as acceptor demonstrated an impressive PCE of 12.70%. Guo et al. developed a series of WBG donor copolymers of PQx-1 (Eg = 1.87 eV), PQx-2 (Eg =1.90 eV), PQx-3 (Eg =1.92 eV) and PQx-4 (Eg =2.00 eV), by incorporating thiophene-flanked phenylene as the D block and Qx as the A unit to realize large Voc and Jsc in NF-PSCs [181]. After fluorination of the phenylene ring, the backbone planarity was improved through intramolecular noncovalent S…F and/or H…F interactions. The PQx-3:IDIC blend showed an efficient exciton dissociation and transfer even under a small energy offset of 0.16 eV, generating the high PCE of 9.7%. Further fluorination would made polymer PQx-4 show increased chain-twisting, mismatched frontier molecular orbital levels with IDIC and decreased PCE of 2.93%. Our group fused two Qx units together to develop a new building block of NQx, which was copolymerized with BDT derivative to afford two WBG copolymers of PBDT-NQxT (Eg = 1.80 eV) and PBDTS-NQxT (Eg = 1.81 eV) [182]. By introducing noctylthienyl side chain onto the NQx skeleton, the π-π* transitions of the resulting NQx-based copolymers were significantly enhanced, contributing to the enhanced absorptions in short wavelength region. A good complementary absorption was then realized when blended with the LBG acceptor of ITIC. PBDTS-NQx exhibited lower-lying HOMO level and higher crystallinity than PBDT-NQxT. Therefore, PBDTS-NQx:ITIC devices produced a higher PCE of 11.47% than that of PBDT-NQx:ITIC devices (9.11%). The chemical structures of Qx-based copolymers are presented in Fig. 9, and the corresponding NF-PSC photovoltaic parameters are summarized in Table 8.
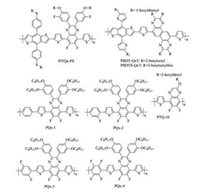
|
Download:
|
| Fig. 9. Chemical structures of Qx based WBG copolymers. | |
|
|
Table 8 Polymer characteristics and the corresponding device performance of NF-OSCs. |
3.7. Thiazolothiazole/bithiazole- and thiadiazole/oxadiazole-based copolymers
Some five-membered heterocycles, such as thiazole (TZ), thiadiazole (TDZ) and oxadiazole (ODZ), are common electron-accepting units due to electron-withdrawing property of imine (C=N). Several TZ derivatives, such as thiazolothiazole (TTz) and bithiazole (BTz) have also been widely introduced into organic semiconductors, which exhibited high performance in organic electronic devices. TTz has a rigid and coplanar fused ring, and thereby may facilitate highly extended π-electron system and strong π-stacking behavior. In 2011, Jenekhe et al. developed TTz-based WBG copolymer of PSEHTT (Eg =1.82 eV) [183], which realized a high PCE of 4.81% in all-PSCs [184, 185] and 8.10% IN BFI-based solar cells [186]. Hou et al. developed two TTz-based copolymers of PBT-TTz (Eg =1.95 eV) and PBT-S-TTz (Eg =1.95 eV) using BDT derivatives as the D blocks [187]. By replacing the alkyl side chain of PBTTTz with the alkylthiol side chain, the HOMO level was lowered to -5.45 eV and a high PCE of 8.22% was obtained in PBT-S-TTz:ITIC devices with a Voc of 0.971 V. Replacing the 2- ethylhexylthienyl sided chains with 2-butyloctylthienyl side chains on BDT unit, Zhang et al. developed the copolymer of PTZ1 (Eg = 1.97 eV) [188]. Using IDIC as the acceptor, a PCE of 11.5% was achieved. Furthermore, the PSCs based on PTZ1:IDIC still exhibited high PCE of 9.6% even with the active layer thickness of 210 nm and a PCE of 10.5% with the device area of 0.81 cm2. When blended with a WBG acceptor of PMI-F-PMI, the LUMO and HOMO offsets between such donor and acceptor materials are as small as 0.08 and 0.19 eV, respectively, giving rise to an extremely high Voc of 1.3 V with a good PCE of 6.0% [189]. The sulfurated analogue PSTZ exhibited lower HOMO level than PTZ1, leading to the improved Voc to 1.00 V in PSTZ:IDIC devices [190]. However, a moderate PCE of 8.13% was obtained due to the large domains that prevented the effective exciton separation. This could be circumvented by adding a small amount ITIC. Thus, the significantly improved PCE of 11.1% was achieved. Using 4, 5-dialkylthienyl side chains on BDT unit and flaking the alkylthiophene bridge facing the TTz unit, the resulting copolymer PTZ-DO (Eg =1.98 eV) showed decreased crystallinity and a moderate PCE [191]. While using alkylphenyl side chains, the crystallinity of PTZP (Eg =2.01 eV) would be enhanced, producing a high PCE of 11.8% in PTZP:IDIC devices [192]. Benzo[1, 2-d:4, 5-d']bis(thiazole) (BBT), which fused a benzene ring into the TTz backbone, has an expanded conjugation for better crystallinity and higher charge carrier mobility. A new WBG polymer PBB-T based on BBT unit was developed by Yang et al. [193]. PBB-T had a large bandgap of 2.10 eV and low HOMO of -5.40 eV. Matched PBB-T with ITIC-F, a high PCE of 13.3 was achieved, showing the potential applications of BBT based polymers. Compared with the rigid TTz and BBT block, BTz has a relatively flexible backbone. However, the double noncovalent conformational lock of N…S enables BTz with a planar structure, which is an interesting molecular design concept of donor polymers. BTz based WBG polymers (PBDTBTz, PTZ6) exhibited the PCEs up to 10% but still needed to be improved further via molecular design and device engineering [194, 195]. Compared with the TZ skeleton, the two electron-deficient imine groups (C=N) in TDZ and ODZ resulted in higher electron affinity and multiple heteroatom characteristics. Our group developed two TDZ-based copolymers of PBDT-TDZ (Eg =2.07 eV) and PBDTS-TDZ (Eg =2.09 eV) [196]. Using ITIC as the acceptor, the resulting single-junction devices based on PBDTS-TDZ exhibited a high Voc of 1.10 V and a small Eloss of 0.48 eV, and produced a high PCE of 12.80% just processed with a single green solvent of o-xylene without any post-treatment. When employing a homo-tandem structure, the tandem cells based on PBDTS-TDZ showed the significantly elevated PCE of 13.35% with a very high Voc of 2.13 V. Using IT- 4 F as the acceptor and modifying the interface with a self-doping small molecular conjugated electrolyte (OTF), the single-junction device performance was further improved to 13.21% [197]. We further developed an ODZ-based copolymer of PBDT-ODZ with a larger bandgap of 2.12 eV [198]. When using ITIC-Th as the NFA, the resulting PSCs showed a high Voc of 1.08 V and a small Eloss of 0.50 eV. The PSCs without any treatments exhibited a PCE of 10.12% due to the poor crystallinity and phase separation. Interestingly, by adding a small amount CuI as the additive, such coordination effect would improve the crystallinity significantly, inducing the fibril-like phase separation and an enhanced PCE of 12.34%. The chemical structures of TTz/BTz- and TDZ/ODZ-based copolymers are presented in Fig. 10, and the corresponding NF-PSC photovoltaic parameters are summarized in Table 9.
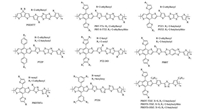
|
Download:
|
| Fig. 10. Chemical structures of TTz/BTz and TDZ/ODZ based WBG copolymers. | |
|
|
Table 9 Polymer characteristics and the corresponding device performance of NF-OSCs. |
3.8. Others
In addition to the above summarized acceptor building blocks, there still has many other units employed to construct WBG copolymers (Fig. 11). Yang et al. combined the N-alkyl-2, 2'- bithiophene-3, 3'-dicarboximide (BTI) with BDT units, and developed PBTIBDTT (Eg = 1.84 eV) and PBTINBTT-S (Eg = 1.83 eV) [199, 200]. PBTINBTT:ITIC-F devices exhibited a high PCE of 11.19% (Table 10). The devices could maintain over 9% PCEs when the active layer thickness varied in a broad range from 70 nm to 350 nm. More importantly, non-fullerene OPV modules were achieved via laser ablation technique and delivered a record PCE of 8.60% (with an active area of 3.48 cm2) for large-area OPV modules [199]. They studied the relationship between the molecular weight (Mn) of PBTINBTT-S and device performance systematically [200]. The PCEs increased gradually from 5.86% to 10.55% along with the increment of Mn from 12 kDa to 38 kDa, due to the substantial enhancement in Jsc and the slight increment in FF. Guo et al. synthesized two phthalimide-based WBG polymers TPhI-BDT (Eg =2.00 eV) and TffPhI-DBT (Eg =2.03 eV) [201]. The fluorine addition enabled TffPhI-DBT with comparable absorption but lower-lying frontier molecular orbitals versus the non-fluorinated analogue TPhI-BDT, contributing to the enhanced PCE from 8.31% to 9.48%. Xia et al. developed two alternating copolymers, PBDT-TPTI (Eg =1.98 eV) and PDTBDT-TPTI (Eg =2.02 eV), derived from BDT or DTBDT with pentacyclic aromatic lactam of N, N-didodecylthieno [2', 3'-d:5, 6]pyrido[3, 4-g]thieno[3, 2-c]-iso-quinoline-5, 11-dione (TPTI) [202]. They found DTBDT block had the more twisted structure, which lowered the packing order of PDTBDT-TPTI, thus reduced PCE from 7.58% to 6.91%. Yang et al. developed a series of random polymers of PTTI-Tx (x = 100, 70, 50, 30) based on TPTI, thiophene and bithiophene units, which improved the PCE successfully from 9.10% to 11.02%, showing the promising strategy of random polymerization [203]. Park et al. reported two pyrrolo [3, 4-c]pyrrole-1, 3-dione (PPD)-based polymers P(T-TPPDT) (Eg =2.02 eV) and P(Tt-TPPDT) (Eg =2.00 eV) [204]. The extended π-conjugation and higher planarity of P(Tt-TPPDT) resulted in improved absorption and higher PCE of 6.35% compared to that of P (T-TPPDT) (PCE = 5.32%). Hou et al. designed and synthesized two new conjugated polymers, PBDT-DTN and PIDT-DTN, based on the naphtho[2, 3-c]thiophene-4, 9-dione (DTN) [205]. They found both polymers could work well with PC71BM (PCE of 7.2% for PBDT-DTN vs. 4.9% for PIDT-DTN). However, in fullerene-free devices, due to the serious differences in charge carrier mobility, exciton dissociation efficiency and charge recombination, the PBDT-DTN:ITIC based devices showed much higher PCE than that of PIDT-DTN:ITIC (8.3% vs. 1.1%). In-depth studies showed that PBDT-DTN had more planar conjugated chain than PIDT-DTN, which could result in stronger molecular interaction, so that proper morphology and better efficiency could be achieved in both fullerene-based and fullerene-free PSCs.

|
Download:
|
| Fig. 11. Chemical structures of other WBG copolymers. | |
|
|
Table 10 Polymer characteristics and the corresponding device performance of NF-OSCs. |
4. Conclusion and outlook
NF-PSCs have shown great potentials in improving device efficiency and long-term operational stability than the traditional fullerene-based OSCs. With the respectable progress after the emergence of LBG NFAs, the development of matched WBG donor copolymers has witnessed explosive growth by rational material design discussed above within the past five years. According to the polymer backbones, the WBG polymers were classified D-type and D—A type and further summarized on the basis of acceptor units. The chemical modifications and the resulting device performances of wide bandgap donor polymers were discussed in detail to better understand the structure-property correlations. To conclude, the results clearly showed that D—A type WBG polymers have more potentials to achieve higher performance in NF-PSCs. The combination of D—A type WBG donor polymers and LBG NFAs has put forward the PCEs of single-junction NF-PSCs over 15% and tandem devices over 17%, showing the bright future for commercialization of PSCs. Based on the above discussions, the universal methods to design high-performance WBG polymers are summarized as follows: 1) Novel donor and acceptor blocks with good absorptions are still highly demanded for developing D—A type WBG polymers to meet higher performance requirements; 2) Rational choice of a matched donor (or acceptor) block for a new acceptor (or donor) block is crucial for tuning the intramolecular charge transfer (ICT) effect to obtain high absorption, desirable energy levels and good crystallinity; 3) Subtle sidechain engineering, such as varying the side chain length, branching point, introducing fluorine, chlorine, sulfur or silicon atoms on the two dimensional side chains, could fine-tune the solubility, crystallinity and energy levels; 4) For a new designed WBG polymer, pairing with matched NFAs and combining with sophisticated device engineering (device structure, interfacial contact, morphology control, etc.) would help to maximize the device performance.
On the other hand, it is worth note that pursuing of a much higher PCE is still the main focus in this field. In most cases, the complicated synthetic route followed by multiple purification procedure vastly push up the costs, and the use of high toxic solvents is also detrimental to the environment and human health, which run counter to the original "green energy" purpose. Therefore, maintaining the high performance in large-scale and long-term devices is still one of the main challenges, which prevents their practical applications. To our excitement, the aims of lowering synthetic costs and using low toxic solvents have been focused by many researchers. However, developing scalable and long-term highly efficient devices to realize the ultimate goal of renewable energy still have a long way.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21825502, 51573107, 91633301 and 21432005) and the Foundation of State Key Laboratory of Polymer Materials Engineering of China (No. sklpme2017-2-04).
| [1] |
H.W. Hu, P.C.Y. Chow, G.Y. Zhang, et al., Acc. Chem. Res. 50 (2017) 2519-2528. DOI:10.1021/acs.accounts.7b00293 |
| [2] |
G.Y. Zhang, J.B. Zhao, P.C.Y. Chow, et al., Chem. Rev. 118 (2018) 3447-3507. DOI:10.1021/acs.chemrev.7b00535 |
| [3] |
Y.M. Sun, H.T. Fu, Z.H. Wang, Angew. Chem. Int. Ed. 58 (2019) 4442-4453. DOI:10.1002/anie.201806291 |
| [4] |
S.S. Li, L. Ye, W.C. Zhao, et al., J. Am. Chem. Soc. 140 (2018) 7159-7167. DOI:10.1021/jacs.8b02695 |
| [5] |
S.Q. Zhang, Y.P. Qin, J. Zhu, J.H. Hou, Adv. Mater. 30 (2018) 1800868. DOI:10.1002/adma.v30.20 |
| [6] |
H. Zhang, H.F. Yao, J.X. Hou, et al., Adv. Mater. 30 (2018) 1800613. DOI:10.1002/adma.v30.28 |
| [7] |
B. Kan, H.R. Feng, H.F. Yao, et al., Sci. China Chem. 61 (2018) 1307-1313. DOI:10.1007/s11426-018-9334-9 |
| [8] |
X.P. Xu, Y. Li, M.M. Luo, Q. Peng, Chin. Chem. Lett. 27 (2016) 1241-1249. DOI:10.1016/j.cclet.2016.05.006 |
| [9] |
S.S. Chen, S.M. Lee, J.Q. Xu, et al., Energy Environ. Sci. 11 (2018) 2569-2580. DOI:10.1039/C8EE01546E |
| [10] |
Y.H. Cai, L.J. Huo, Y.M. Sun, Adv. Mater. 29 (2017) 1605437. DOI:10.1002/adma.v29.22 |
| [11] |
J.B. Zhao, Y.K. Li, G.F. Yang, et al., Nat. Energy 1 (2016) 15027. DOI:10.1038/nenergy.2015.27 |
| [12] |
T. Liu, X.X. Pan, X.Y. Meng, et al., Adv. Mater. 29 (2017) 1604251. DOI:10.1002/adma.201604251 |
| [13] |
L.J. Liu, J.N. Pei, S.P. Wen, et al., Macromol. Chem. Phys. 214 (2013) 1836-1844. DOI:10.1002/macp.v214.16 |
| [14] |
K. Feng, G.F. Yang, X.P. Xu, et al., Adv. Energy Mater. 8 (2018) 1602773. DOI:10.1002/aenm.v8.6 |
| [15] |
L.J. Huo, X.N. Xue, T. Liu, et al., Chem. Mater. 30 (2018) 3294-3300. DOI:10.1021/acs.chemmater.8b00510 |
| [16] |
T. Liu, L.J. Huo, S. Chandrabose, et al., Adv. Mater. 30 (2018) 1707353. DOI:10.1002/adma.201707353 |
| [17] |
Fraga Domínguez I., A. Distler, L. Lüer, Adv. Energy Mater. 7 (2017) 1601320. DOI:10.1002/aenm.201601320 |
| [18] |
F.G. Shen, J.Z. Xu, X.M. Li, C.L. Zhan, J. Mater. Chem. A 6 (2018) 15433-15455. DOI:10.1039/C8TA04718A |
| [19] |
J.H. Hou, Z.A. Tan, Y. Yan, et al., J. Am. Chem. Soc. 128 (2006) 4911-4916. DOI:10.1021/ja060141m |
| [20] |
X. Guo, M.J. Zhang, C.H. Cui, J.H. Hou, Y.F. Li, ACS Appl. Mater. Interfaces 6 (2014) 8190-8198. DOI:10.1021/am500836u |
| [21] |
M.T. Dang, L. Hirsch, G. Wantz, Adv. Mater. 23 (2011) 3597-3602. DOI:10.1002/adma.201100792 |
| [22] |
X. Guo, C.H. Cui, M.J. Zhang, et al., Energy Environ. Sci. 5 (2012) 7943-7949. DOI:10.1039/c2ee21481d |
| [23] |
G.J. Zhao, Y.J. He, Y.F. Li, Adv. Mater. 22 (2010) 4355-4358. DOI:10.1002/adma.v22:39 |
| [24] |
S.X. Li, W.Q. Liu, M.M. Shi, et al., Energy Environ. Sci. 9 (2016) 604-610. DOI:10.1039/C5EE03481G |
| [25] |
B. Xiao, A.L. Tang, J. Yang, Z.X. Wei, E.J. Zhou, ACS Macro Lett. 6 (2017) 410-414. DOI:10.1021/acsmacrolett.7b00097 |
| [26] |
S. Holliday, R.S. Ashraf, A. Wadsworth, et al., Nat. Commun. 7 (2016) 11585. DOI:10.1038/ncomms11585 |
| [27] |
D. Baran, R.S. Ashraf, D.A. Hanifi, et al., Nat. Mater. 16 (2016) 363. |
| [28] |
J.H. Hou, L.J. Huo, C. He, C.H. Yang, Y.F. Li, Macromolecules 39 (2006) 594-603. DOI:10.1021/ma051883n |
| [29] |
M.J. Zhang, X. Guo, W. Ma, H. Ade, J.H. Hou, Adv. Mater. 26 (2014) 5880-5885. DOI:10.1002/adma.v26.33 |
| [30] |
C.G. Park, G.E. Park, J.H. Lee, et al., Polymer 146 (2018) 142-150. DOI:10.1016/j.polymer.2018.05.027 |
| [31] |
X.X. Liao, X.G. Zhao, Z.G. Zhang, et al., Sol. Energy Mater. Sol. C 117 (2013) 336-342. DOI:10.1016/j.solmat.2013.06.035 |
| [32] |
P.P. Khlyabich, A.E. Rudenko, B.C. Thompson, Polym. Sci. J., Part. A:Polym. Chem. 52 (2014) 1055-1058. DOI:10.1002/pola.27095 |
| [33] |
X.E. Jia, Z.M. Chen, C.H. Duan, et al., J. Mater. Chem. C 7 (2019) 314-323. DOI:10.1039/C8TC04746D |
| [34] |
H. Zhang, S.S. Li, B.W. Xu, et al., J. Mater. Chem. A 4 (2016) 18043-18049. DOI:10.1039/C6TA07672F |
| [35] |
Y. Qin, M.A. Uddin, Y. Chen, et al., Adv. Mater. 28 (2016) 9416-9422. DOI:10.1002/adma.201601803 |
| [36] |
J. Hou, M.H. Park, S. Zhang, et al., Macromolecules 41 (2008) 6012-6018. DOI:10.1021/ma800820r |
| [37] |
H.F. Yao, L. Ye, H. Zhang, et al., Chem. Rev. 116 (2016) 7397-7457. DOI:10.1021/acs.chemrev.6b00176 |
| [38] |
T.E. Kang, T. Kim, C. Wang, S. Yoo, B.J. Kim, Chem. Mater. 27 (2015) 2653-2658. DOI:10.1021/acs.chemmater.5b00481 |
| [39] |
J.H. Kim, J.B. Park, S.C. Yoon, I.H. Jung, D.H. Hwang, J, . Mater. Chem. C 4 (2016) 2170-2177. DOI:10.1039/C5TC04449A |
| [40] |
G.D. Li, Q.Q. Xu, C.M. Chang, et al., Macromol. Rapid Commun. 39 (2018) 1800660. |
| [41] |
D. Hao, M. Li, Y.H. Liu, C.H. Li, Z.S. Bo, Dyes Pigments 162 (2019) 120-125. DOI:10.1016/j.dyepig.2018.09.079 |
| [42] |
Y.K. An, X.F. Liao, L. Chen, et al., Adv. Funct. Mater. 28 (2018) 1706517. DOI:10.1002/adfm.v28.16 |
| [43] |
X.F. Liao, Z.Y. Yao, K. Gao, et al., Adv. Energy Mater. 8 (2018) 1801214. DOI:10.1002/aenm.201801214 |
| [44] |
M.J. Cho, J. Seo, K.H. Kim, D.H. Choi, P.N. Prasad, Macromol.Rapid Commun. 33 (2012) 146-151. DOI:10.1002/marc.201100501 |
| [45] |
M.J. Cho, J. Seo, K. Luo, et al., Polymer 53 (2012) 3835-3841. DOI:10.1016/j.polymer.2012.07.007 |
| [46] |
J. Wolf, F. Cruciani, El Labban A., P.M. Beaujuge, Chem. Mater. 27 (2015) 4184-4187. DOI:10.1021/acs.chemmater.5b01520 |
| [47] |
Y. Firdaus, L.P. Maffei, F. Cruciani, et al., Adv. Energy Mater. 7 (2017) 1700834. DOI:10.1002/aenm.201700834 |
| [48] |
G.E. Park, S. Choi, S.Y. Park, et al., Adv. Energy Mater. 7 (2017) 1700566. DOI:10.1002/aenm.201700566 |
| [49] |
Y.H. Liu, H. Lu, M. Li, et al., Macromolecules 51 (2018) 8646-8651. DOI:10.1021/acs.macromol.8b01677 |
| [50] |
D.D. Xia, Y. Wu, Q. Wang, et al., Macromolecules 49 (2016) 6445-6454. DOI:10.1021/acs.macromol.6b01326 |
| [51] |
R. Kong, Z. Xiao, F.Y. Xie, J.X. Jiang, L.M. Ding, New. J. Chem. 41 (2017) 2895-2898. DOI:10.1039/C6NJ03991J |
| [52] |
D.L. Liu, B. Yang, B. Jang, et al., Energy Environ. Sci. 10 (2017) 546-551. DOI:10.1039/C6EE03489F |
| [53] |
X.J. Zhang, J.V. Zhang, H. Lu, et al., J. Mater. Chem. C 3 (2015) 6979-6985. DOI:10.1039/C5TC01148E |
| [54] |
J.C. Zhang, H.M. Xiao, X.J. Zhang, et al., J. Mater. Chem. C 4 (2016) 5656-5663. DOI:10.1039/C6TC01438K |
| [55] |
H.F. Yao, R.N. Yu, T.J. Shin, et al., Adv. Energy Mater. 6 (2016) 1600742. DOI:10.1002/aenm.201600742 |
| [56] |
H. Yang, Y. Wu, Y. Zou, et al., J. Mater. Chem. A 6 (2018) 14700-14708. DOI:10.1039/C8TA05207G |
| [57] |
H. Lu, M. Li, Z.Z. Bi, et al., Org. Electron. 65 (2019) 419-425. DOI:10.1016/j.orgel.2018.11.040 |
| [58] |
X. Gong, S.Y. Feng, G.W. Li, et al., Dyes Pigments 141 (2017) 342-347. DOI:10.1016/j.dyepig.2017.02.022 |
| [59] |
Y.H. Liu, S.S. Chen, G.Y. Zhang, P.C.Y. Chow, H. Yan, J. Mater. Chem. A 5 (2017) 15017-15020. DOI:10.1039/C7TA03600K |
| [60] |
S.S. Chen, H.T. Yao, Z.K. Li, et al., Adv. Energy Mater. 7 (2017) 1602304. DOI:10.1002/aenm.201602304 |
| [61] |
H.Y. Jiang, Z. Wang, L.J. Zhang, et al., ACS Appl. Mater. Interfaces 9 (2017) 36061-36069. DOI:10.1021/acsami.7b10059 |
| [62] |
D.S. Tang, J.H. Wan, X.P. Xu, et al., Nano Energy 53 (2018) 258-269. DOI:10.1016/j.nanoen.2018.08.059 |
| [63] |
M. Deng, X.P. Xu, Y.W. Lee, et al., ACS Appl. Mater. Interfaces 11 (2019) 3308-3316. DOI:10.1021/acsami.8b18493 |
| [64] |
H.J. Bin, L. Zhong, Z.G. Zhang, et al., Sci. China Chem. 59 (2016) 1317-1322. |
| [65] |
T.H. Yan, H.J. Bin, C.K. Sun, Z.G. Zhang, Y.F. Li, Org. Electron. 55 (2018) 106-111. DOI:10.1016/j.orgel.2018.01.018 |
| [66] |
T.H. Yan, H.J. Bin, C.K. Sun, Z.G. Zhang, Y.F. Li, Org. Electron. 57 (2018) 255-262. DOI:10.1016/j.orgel.2018.03.028 |
| [67] |
L. Gao, Z.G. Zhang, L.W. Xue, et al., Adv. Mater. 28 (2016) 1884-1890. DOI:10.1002/adma.201504629 |
| [68] |
L. Gao, Z.G. Zhang, H.J. Bin, et al., Adv. Mater. 28 (2016) 8288-8295. DOI:10.1002/adma.201601595 |
| [69] |
Y.X. Li, L. Zhong, F.P. Wu, et al., Energy Environ. Sci. 9 (2016) 3429-3435. DOI:10.1039/C6EE00315J |
| [70] |
H.J. Bin, Z.G. Zhang, L. Gao, et al., J. Am. Chem. Soc. 138 (2016) 4657-4664. DOI:10.1021/jacs.6b01744 |
| [71] |
R.N. Yu, S.Q. Zhang, H.F. Yao, et al., Adv. Mater. 29 (2017) 1700437. DOI:10.1002/adma.v29.26 |
| [72] |
Q.P. Fan, W.Y. Su, X.Y. Meng, et al., Sol. RRL 1 (2017) 1700020. DOI:10.1002/solr.v1.5 |
| [73] |
Q.P. Fan, W.Y. Su, X. Guo, et al., J. Mater. Chem. A 5 (2017) 9204-9209. DOI:10.1039/C7TA02075A |
| [74] |
W.Y. Su, G.W. Li, Q.P. Fan, et al., J. Mater. Chem. A 7 (2018) 2351-2359. |
| [75] |
Y.K. Yang, Z.G. Zhang, H.J. Bin, et al., J. Am. Chem. Soc. 138 (2016) 15011-15018. DOI:10.1021/jacs.6b09110 |
| [76] |
H. Huang, H.J. Bin, Z.X. Peng, et al., Macromolecules 51 (2018) 6028-6036. DOI:10.1021/acs.macromol.8b01036 |
| [77] |
H.J. Bin, L. Gao, Z.G. Zhang, et al., Nat. Commun. 7 (2016) 13651. DOI:10.1038/ncomms13651 |
| [78] |
H.J. Bin, Y.K. Yang, Z.X. Peng, et al., Adv. Energy Mater. 8 (2018) 1702324. DOI:10.1002/aenm.201702324 |
| [79] |
H.J. Bin, L. Zhong, Y.K. Yang, et al., Adv. Energy Mater. 7 (2017) 1700746. DOI:10.1002/aenm.201700746 |
| [80] |
L.W. Xue, Y.K. Yang, J.Q. Xu, et al., Adv. Mater. 29 (2017) 1703344. DOI:10.1002/adma.201703344 |
| [81] |
W.R. Liu, J.Y. Zhang, Z.C. Zhou, et al., Adv. Mater. 30 (2018) 1800403. DOI:10.1002/adma.v30.26 |
| [82] |
B.B. Fan, P. Zhu, J.M. Xin, et al., Adv. Energy Mater. 8 (2018) 1703085. DOI:10.1002/aenm.v8.14 |
| [83] |
Z. Liu, D.Y. Liu, K.L. Zhang, et al., J. Mater. Chem. A 5 (2017) 21650-21657. DOI:10.1039/C7TA07390A |
| [84] |
D.Y. Liu, K.L. Zhang, Y.Q. Zhong, et al., J. Mater. Chem. A 6 (2018) 18125-18132. DOI:10.1039/C8TA07134A |
| [85] |
Y.Q. Zhong, D.Y. Liu, K.L. Zhang, et al., J. Polym. Sci.Part. A:Polym. Chem. 56 (2018) 2762-2770. DOI:10.1002/pola.v56.24 |
| [86] |
S.S. Chen, Y.H. Liu, L. Zhang, et al., J. Am. Chem. Soc. 139 (2017) 6298-6301. DOI:10.1021/jacs.7b01606 |
| [87] |
K. Feng, J. Yuan, Z.Z. Bi, et al., iScience 12 (2019) 1-12. DOI:10.1016/j.isci.2018.12.027 |
| [88] |
A.L. Tang, B. Xiao, F. Chen, et al., Adv. Energy Mater. 8 (2018) 1801582. DOI:10.1002/aenm.v8.25 |
| [89] |
W.Y. Su, Y. Meng, X. Guo, et al., J. Mater. Chem. A 6 (2018) 16403-16411. DOI:10.1039/C8TA05376F |
| [90] |
W.C. Zhao, S.Q. Zhang, Y. Zhang, et al., Adv. Mater. 30 (2018) 1704837. DOI:10.1002/adma.v30.4 |
| [91] |
Z.T. Liu, Y.R. Gao, J. Dong, et al., J. Phys. Chem. Lett. 9 (2018) 6955-6962. DOI:10.1021/acs.jpclett.8b03247 |
| [92] |
S.J. Liu, Y. Firdaus, S. Thomas, et al., Angew. Chem. Int. Ed. 57 (2018) 531-535. DOI:10.1002/anie.201709509 |
| [93] |
N. Bauer, Q.Q. Zhang, J.S. Zhu, et al., J. Mater. Chem. A 5 (2017) 22536-22541. DOI:10.1039/C7TA07882J |
| [94] |
Y.Z. Lin, F.W. Zhao, S.K.K. Prasad, et al., Adv. Mater. 30 (2018) 1706363. DOI:10.1002/adma.201706363 |
| [95] |
N. Bauer, Q.Q. Zhang, J.B. Zhao, et al., J. Mater. Chem. A 5 (2017) 4886-4893. DOI:10.1039/C6TA10450A |
| [96] |
Y.Y. Gao, Z. Wang, J.Q. Zhang, et al., Macromolecules 51 (2018) 2498-2505. DOI:10.1021/acs.macromol.7b02676 |
| [97] |
Z.J. Li, X.P. Xu, G.J. Zhang, et al., Sol. RRL 2 (2018) 1800186. DOI:10.1002/solr.v2.10 |
| [98] |
Y.Y. Gao, Z. Wang, J.Q. Zhang, et al., J. Mater. Chem. A 6 (2018) 4023-4031. DOI:10.1039/C7TA10976H |
| [99] |
Y.Y. Gao, R.X. Zhu, Z. Wang, et al., ACSAppl. Energy Mater. 1 (2018) 1888-1892. DOI:10.1021/acsaem.8b00574 |
| [100] |
W.B. Li, G.D. Li, X. Guo, et al., J. Mater. Chem. A 5 (2017) 19680-19686. DOI:10.1039/C7TA06476D |
| [101] |
Z.J. Li, X.F. Xu, W. Zhang, et al., Energy Environ. Sci. 10 (2017) 2212-2221. DOI:10.1039/C7EE01858D |
| [102] |
W.K. Zhong, J. Cui, B.B. Fan, et al., Chem. Mater. 29 (2017) 8177-8186. DOI:10.1021/acs.chemmater.7b02228 |
| [103] |
X. Liu, C. Zhang, C. Duan, et al., J. Am. Chem. Soc. 140 (2018) 8934-8943. DOI:10.1021/jacs.8b05038 |
| [104] |
W. Huang, M.L. Li, F.Y. Lin, et al., Mol. Syst. Des. Eng. 3 (2018) 103-112. DOI:10.1039/C7ME00088J |
| [105] |
W.C. Chen, H.X. Jiang, G.Y. Huang, et al., Sol. RRL 2 (2018) 1800101. DOI:10.1002/solr.v2.8 |
| [106] |
W.B. Li, G.D. Li, X. Guo, et al., J. Mater. Chem. A 6 (2018) 6551-6558. DOI:10.1039/C7TA11059F |
| [107] |
S.G. Wen, W.C. Chen, G.Y. Huang, et al., J. Mater. Chem. C 6 (2018) 1753-1758. DOI:10.1039/C7TC04750A |
| [108] |
W.C. Chen, G.Y. Huang, X.M. Li, et al., ACS Appl. Mater. Interfaces 10 (2018) 42747-42755. DOI:10.1021/acsami.8b16554 |
| [109] |
Z.Y. Li, B.B. Fan, B.T. He, et al., Sci. China Chem. 61 (2018) 427-436. DOI:10.1007/s11426-017-9188-7 |
| [110] |
C. Weng, W.G. Wang, J.T. Liang, et al., J. Polym. Sci. Part. A:Polym. Chem. 56 (2018) 2330-2343. DOI:10.1002/pola.v56.20 |
| [111] |
Y. Zou, Y.Y. Dong, Y. Wu, et al., Org. Electron. 63 (2018) 289-295. DOI:10.1016/j.orgel.2018.09.047 |
| [112] |
M. He, W.L. Li, H.K. Tian, et al., Org. Electron. 65 (2019) 31-38. DOI:10.1016/j.orgel.2018.10.034 |
| [113] |
H.R. Lin, S.S. Chen, Z.K. Li, et al., Adv. Mater. 27 (2015) 7299-7304. DOI:10.1002/adma.201502775 |
| [114] |
J. Liu, L.K. Ma, Z.K. Li, et al., J. Mater. Chem. A 5 (2017) 22480-22488. DOI:10.1039/C7TA07830G |
| [115] |
H.W. Hu, K. Jiang, P.C.Y. Chow, et al., Adv. Energy Mater. 8 (2018) 1701674. DOI:10.1002/aenm.201701674 |
| [116] |
Y.K. An, X.F. Liao, L. Chen, et al., Sol. RRL 2 (2018) 1800291. |
| [117] |
D.P. Qian, L. Ye, M.J. Zhang, et al., Macromolecules 45 (2012) 9611-9617. DOI:10.1021/ma301900h |
| [118] |
L. Ye, W. Jiang, W.C. Zhao, et al., Small 10 (2014) 4658-4663. DOI:10.1002/smll.v10.22 |
| [119] |
W.C. Zhao, D.P. Qian, S.Q. Zhang, et al., Adv. Mater. 28 (2016) 4734-4739. DOI:10.1002/adma.v28.23 |
| [120] |
X.H. Liu, Y. Zou, H.Q. Wang, et al., ACS Appl. Mater. Interfaces 10 (2018) 38302-38309. DOI:10.1021/acsami.8b15028 |
| [121] |
Z.G. Zhang, Y. Yang, J. Yao, et al., Angew. Chem. Int. Ed. 56 (2017) 13503-13507. DOI:10.1002/anie.201707678 |
| [122] |
Y. Wu, Y. Zou, H. Yang, et al., ACS Appl. Mater. Interfaces 9 (2017) 37078-37086. DOI:10.1021/acsami.7b11488 |
| [123] |
Z.P. Fei, F.D. Eisner, X.C. Jiao, et al., Adv. Mater. 30 (2018) 1705209. DOI:10.1002/adma.v30.8 |
| [124] |
Y.N. Wu, C.B. An, L.B. Shi, et al., Angew. Chem. Int. Ed. 57 (2018) 12911-12915. DOI:10.1002/anie.201807865 |
| [125] |
Y. Wu, H. Yang, Y. Zou, et al., Sol. RRL 2 (2018) 1800060. DOI:10.1002/solr.v2.7 |
| [126] |
L.L. Ye, Y.P. Xie, K.K. Weng, et al., Nano Energy 58 (2019) 220-226. DOI:10.1016/j.nanoen.2019.01.039 |
| [127] |
Y. Wang, Q.P. Fan, X. Guo, et al., J. Mater. Chem. A 5 (2017) 22180-22185. DOI:10.1039/C7TA07785H |
| [128] |
Q.P. Fan, Y. Wang, M.J. Zhang, et al., Adv. Mater. 30 (2018) 1704546. DOI:10.1002/adma.201704546 |
| [129] |
S.S. Li, L. Ye, W.C. Zhao, et al., Adv. Mater. 29 (2017) 1704051. DOI:10.1002/adma.201704051 |
| [130] |
Q.P. Fan, W.Y. Su, Y. Wang, et al., Sci. China Chem. 61 (2018) 531-537. DOI:10.1007/s11426-017-9199-1 |
| [131] |
Z. Zheng, Q. Hu, S.Q. Zhang, et al., Adv. Mater. (2018) 1801801. |
| [132] |
T. Liu, Z.H. Luo, Q.P. Fan, et al., Energy Environ. Sci. 11 (2018) 3275-3282. DOI:10.1039/C8EE01700J |
| [133] |
J.L. Wang, K.K. Liu, L. Hong, et al., ACS Energy Lett. 3 (2018) 2967-2976. DOI:10.1021/acsenergylett.8b01808 |
| [134] |
Q.P. Fan, Q.L. Zhu, Z. Xu, et al., Nano Energy 48 (2018) 413-420. DOI:10.1016/j.nanoen.2018.04.002 |
| [135] |
J. Yuan, Y.Q. Zhang, L.Y. Zhou, et al., Joule 3 (2019) 1140-1151. DOI:10.1016/j.joule.2019.01.004 |
| [136] |
H.T. Yao, F.J. Bai, H.W. Hu, et al., ACS Energy Lett. 4 (2019) 417-422. DOI:10.1021/acsenergylett.8b02114 |
| [137] |
W.C. Zhao, S.S. Li, H.F. Yao, et al., J. Am. Chem. Soc. 139 (2017) 7148-7151. DOI:10.1021/jacs.7b02677 |
| [138] |
Y. Cui, S.Q. Zhang, N.N. Liang, et al., Adv. Mater. (2018) 1802499. |
| [139] |
T. Rehman, Z.X. Liu, T.K. Lau, et al., ACS Appl. Mater. Interfaces (2018), doi: http://dx.doi.org/10.1021/acsami.1028b16628.
|
| [140] |
Q.P. Fan, Z. Xu, X. Guo, et al., Nano Energy 40 (2017) 20-26. DOI:10.1016/j.nanoen.2017.07.047 |
| [141] |
X.N. Xue, K.K. Weng, F. Qi, et al., Adv. Energy Mater. (2018) 1802686. |
| [142] |
W.B. Li, G.D. Li, H. Guo, et al., J. Mater. Chem. A 7 (2019) 1307-1314. DOI:10.1039/C8TA11006A |
| [143] |
Y.P. Qin, L. Ye, S.Q. Zhang, et al., J. Mater. Chem. A 6 (2018) 4324-4330. DOI:10.1039/C8TA00368H |
| [144] |
Y.H. Li, D.Y. Liu, J.Y. Wang, et al., Chem. Mater. 29 (2017) 8249-8257. DOI:10.1021/acs.chemmater.7b02495 |
| [145] |
Y.H. Li, L.R. Duan, D.Y. Liu, et al., J. Mater. Chem. C 6 (2018) 2806-2813. DOI:10.1039/C8TC00148K |
| [146] |
D.Y. Liu, J.Y. Wang, C.Y. Gu, et al., Adv. Mater. 30 (2018) 1705870. DOI:10.1002/adma.v30.8 |
| [147] |
M.H. Hoang, G.E. Park, S. Choi, et al., J. Mater. Chem. C 7 (2019) 111-118. DOI:10.1039/C8TC05035J |
| [148] |
J.Y. Kim, S. Park, S. Lee, et al., Adv. Energy Mater. (2018) 1801601. |
| [149] |
L.J. Huo, T. Liu, X.B. Sun, et al., Adv. Mater. 27 (2015) 2938-2944. DOI:10.1002/adma.v27.18 |
| [150] |
D. Sun, D. Meng, Y.H. Cai, et al., J. Am. Chem. Soc. 137 (2015) 11156-11162. DOI:10.1021/jacs.5b06414 |
| [151] |
D. Meng, D. Sun, C.M. Zhong, et al., J. Am. Chem. Soc. 138 (2016) 375-380. DOI:10.1021/jacs.5b11149 |
| [152] |
Y.Z. Lin, Q. He, F.W. Zhao, et al., J. Am. Chem. Soc. 138 (2016) 2973-2976. DOI:10.1021/jacs.6b00853 |
| [153] |
Y.Z. Lin, F.W. Zhao, Q. He, et al., J. Am. Chem. Soc. 138 (2016) 4955-4961. DOI:10.1021/jacs.6b02004 |
| [154] |
W.T. Xiong, X.Y. Meng, T. Liu, et al., Org. Electron. 50 (2017) 376-383. DOI:10.1016/j.orgel.2017.08.005 |
| [155] |
K.K. Dou, X.C. Wang, Z.R. Du, et al., J. Mater. Chem. A 7 (2018) 958-964. |
| [156] |
T. Liu, D. Meng, Y.H. Cai, et al., Adv. Sci. 3 (2016) 1600117. DOI:10.1002/advs.201600117 |
| [157] |
J.Y. Yuan, W.L. Ma, J. Mater. Chem. A 3 (2015) 7077-7085. DOI:10.1039/C4TA06648K |
| [158] |
T. Kim, J.H. Kim, T.E. Kang, et al., Nat. Commun. 6 (2015) 8547. DOI:10.1038/ncomms9547 |
| [159] |
C. Lee, T. Giridhar, J. Choi, et al., Chem. Mater. 29 (2017) 9407-9415. DOI:10.1021/acs.chemmater.7b03495 |
| [160] |
M.A. Uddin, Y. Kim, R. Younts, et al., Macromolecules 49 (2016) 6374-6383. DOI:10.1021/acs.macromol.6b01414 |
| [161] |
J.Y. Yuan, W.P. Guo, Y.X. Xia, et al., Nano Energy 35 (2017) 251-262. DOI:10.1016/j.nanoen.2017.03.050 |
| [162] |
G.Q. Ding, J.Y. Yuan, F. Jin, et al., Nano Energy 36 (2017) 356-365. DOI:10.1016/j.nanoen.2017.04.061 |
| [163] |
X.F. Xu, Z.J. Li, W. Zhang, et al., Adv. Energy Mater. 8 (2018) 1700908. DOI:10.1002/aenm.v8.1 |
| [164] |
T. Zhang, W.H. Feng, W. Wang, et al., J. Mater. Chem. C 6 (2018) 8418-8428. DOI:10.1039/C8TC02553C |
| [165] |
J. Oh, K. Kranthiraja, C. Lee, et al., Adv. Mater. 28 (2016) 10016-10023. DOI:10.1002/adma.201602298 |
| [166] |
A. Kim, C.G. Park, S.H. Park, et al., J. Mater. Chem. A 6 (2018) 10095-10103. DOI:10.1039/C8TA01765D |
| [167] |
F. Yang, D.P. Qian, A.H. Balawi, et al., Phys. Chem. Chem. Phys. 19 (2017) 23990-23998. DOI:10.1039/C7CP04780K |
| [168] |
W.T. Hadmojo, F.T.A. Wibowo, D.Y. Ryu, I.H. Jung, S.Y. Jang, ACS Appl. Mater. Interfaces 9 (2017) 32939-32945. DOI:10.1021/acsami.7b09757 |
| [169] |
F.Y. Lin, W. Huang, H.T. Sun, et al., Chem. Mater. 29 (2017) 5636-5645. DOI:10.1021/acs.chemmater.7b01335 |
| [170] |
Y. Xie, W. Huang, Q.B. Liang, et al., ACS Energy Lett. (2019) 4-16. |
| [171] |
J. Zhang, G.Y. Huang, H. Tan, et al., Polymer 145 (2018) 108-116. DOI:10.1016/j.polymer.2018.04.069 |
| [172] |
Y.J. Guo, M. Li, Y.Y. Zhou, et al., Macromolecules 50 (2017) 7984-7992. DOI:10.1021/acs.macromol.7b01738 |
| [173] |
L.Y. Lan, Z.M. Chen, Q. Hu, et al., Adv. Sci. 3 (2016) 1600032. DOI:10.1002/advs.201600032 |
| [174] |
B.B. Fan, L. Ying, Z.F. Wang, et al., Energy Environ. Sci. 10 (2017) 1243-1251. DOI:10.1039/C7EE00619E |
| [175] |
B.B. Fan, K. Zhang, X.F. Jiang, et al., Adv. Mater. 29 (2017) 1606396. DOI:10.1002/adma.201606396 |
| [176] |
P. Zhu, B.B. Fan, X.Y. Du, et al., ACS Appl. Mater. Interfaces 10 (2018) 22495-22503. DOI:10.1021/acsami.8b05700 |
| [177] |
K. Li, Z.C. Hu, Z.M.Y. Zeng, et al., Org. Electron. 57 (2018) 317-322. DOI:10.1016/j.orgel.2018.03.005 |
| [178] |
B.B. Fan, X.Y. Du, F. Liu, et al., Nat. Energy 3 (2018) 1051-1058. DOI:10.1038/s41560-018-0263-4 |
| [179] |
J. Yuan, L.X. Qiu, Z.G. Zhang, et al., Nano Energy 30 (2016) 312-320. DOI:10.1016/j.nanoen.2016.10.008 |
| [180] |
C.K. Sun, F. Pan, H.J. Bin, et al., Nat. Commun. 9 (2018) 743. DOI:10.1038/s41467-018-03207-x |
| [181] |
J. Yang, M.A. Uddin, Y.M. Tang, et al., ACS Appl. Mater. Interfaces 10 (2018) 23235-23246. DOI:10.1021/acsami.8b04432 |
| [182] |
T. Yu, X.P. Xu, G.J. Zhang, et al., Adv. Funct. Mater. 27 (2017) 1701491. DOI:10.1002/adfm.v27.28 |
| [183] |
S. Subramaniyan, H. Xin, F.S. Kim, et al., Adv. Energy Mater. 1 (2011) 854-860. DOI:10.1002/aenm.v1.5 |
| [184] |
T. Earmme, Y.J. Hwang, N.M. Murari, S. Subramaniyan, S.A. Jenekhe, J. Am. Chem. Soc. 135 (2013) 14960-14963. DOI:10.1021/ja4085429 |
| [185] |
T. Earmme, Y.J. Hwang, S. Subramaniyan, S.A. Jenekhe, Adv. Mater. 26 (2014) 6080-6085. DOI:10.1002/adma.201401490 |
| [186] |
Y.J. Hwang, H.Y. Li, B.A.E. Courtright, S. Subramaniyan, S.A. Jenekhe, Adv. Mater. 28 (2016) 124-131. DOI:10.1002/adma.201503801 |
| [187] |
K. Zhao, Q. Wang, B.W. Xu, et al., J. Mater. Chem. A 4 (2016) 9511-9518. DOI:10.1039/C6TA03288E |
| [188] |
B. Guo, W.B. Li, X. Guo, et al., Adv. Mater. 29 (2017) 1702291. DOI:10.1002/adma.201702291 |
| [189] |
Y.D. Zhang, X. Guo, B. Guo, et al., Adv. Funct. Mater. 27 (2017) 1603892. DOI:10.1002/adfm.v27.10 |
| [190] |
W.Y. Su, Q.P. Fan, X. Guo, et al., Nano Energy 38 (2017) 510-517. DOI:10.1016/j.nanoen.2017.05.060 |
| [191] |
B.F. Zhao, W.P. Wang, J.M. Xin, et al., ACS Sustain. Chem. Eng. 6 (2018) 2177-2187. DOI:10.1021/acssuschemeng.7b03606 |
| [192] |
X. Guo, W.B. Li, H. Guo, et al., J. Mater. Chem. A 6 (2018) 16529-16536. DOI:10.1039/C8TA05868G |
| [193] |
S.G. Wen, Y. Li, T. Rath, et al., Chem. Mater. 31 (2019) 919-926. DOI:10.1021/acs.chemmater.8b04265 |
| [194] |
B. Guo, W.B. Li, X. Guo, et al., Nano Energy 34 (2017) 556-561. DOI:10.1016/j.nanoen.2017.03.013 |
| [195] |
S.R. Huang, L. Chen, Z.H. Liao, et al., Org. Electron. 64 (2019) 110-116. DOI:10.1016/j.orgel.2018.10.019 |
| [196] |
X.P. Xu, T. Yu, Z.Z. Bi, et al., Adv. Mater. 30 (2018) 1703973. DOI:10.1002/adma.v30.3 |
| [197] |
S.C. Wang, Z.J. Li, X.P. Xu, et al., J. Mater. Chem. A 6 (2018) 22503-22507. DOI:10.1039/C8TA08948E |
| [198] |
X.P. Xu, Z.J. Li, Z.Z. Bi, et al., Adv. Mater. 30 (2018) 1800737. DOI:10.1002/adma.v30.28 |
| [199] |
T. Zhang, G. Zeng, F. Ye, X.L. Zhao, X.N. Yang, Adv. Energy Mater. 8 (2018) 1801387. DOI:10.1002/aenm.v8.25 |
| [200] |
Z. Li, D.L. Yang, T. Zhang, et al., Small 14 (2018) 1704491. DOI:10.1002/smll.v14.16 |
| [201] |
J.W. Yu, J. Yang, X. Zhou, et al., Macromolecules 50 (2017) 8928-8937. DOI:10.1021/acs.macromol.7b01958 |
| [202] |
P.L. Gao, J.F. Tong, P.Z. Guo, et al., J. Polym. Sci.Part. A:Polym. Chem. 56 (2018) 85-95. DOI:10.1002/pola.v56.1 |
| [203] |
S.S. Chen, H.J. Cho, J. Lee, et al., Adv. Energy Mater. 7 (2017) 1701125. DOI:10.1002/aenm.201701125 |
| [204] |
V. Tamilavan, J. Lee, R. Agneeswari, et al., Org. Electron. 63 (2018) 78-85. DOI:10.1016/j.orgel.2018.09.015 |
| [205] |
B. Yang, S.Q. Zhang, Y. Chen, et al., Macromolecules 50 (2017) 1453-1462. DOI:10.1021/acs.macromol.6b02733 |
 2019, Vol. 30
2019, Vol. 30