b College of Chemical and Environmental Engineering, Shandong University of Science and Technology, Qingdao 266590, China;
c State Key Laboratory of Heavy Oil Processing, China University of Petroleum (East China), Qingdao 266580, China
Metal–organic frameworks (MOFs) have emerged as useful materials in gas separation, fluorescence, catalysis, magnetism, drug delivery, and so on [1-4]. MOFs can be readily self-assembled from metal ions/clusters and organic linkers. To achieve predictable structures with new properties, the matching of pre-designed ligand's geometry and metal ion's coordination mode are needed [5-8]. The past researches have found that the construction of MOFs by rigid or flexible carboxylate ligands have different advantages [9-11]. For example, rigid carboxylate ligands are easy to construct robust networks with permanent pores, flexible ones can change their conformations to meet the coordination requirement of metal ions and build more unexpected topologies [12, 13].
As known, heavy metal ions and nitroaromatic compounds (NACs) produced by industrial production have done great damage to the living environment of human beings. However, effective detection of those pollution is a challenging work. There are some reports about detecting the presence of metal ions by different methods. For example, Zhang and Lin's group reported supramolecular compounds including supramolecular polymer, supramolecular organic frameworks, which can detect ions and molecules through different mechanisms [14-18]. Zeng's group reported electrochemical sensing strategy to realize the highly sensitive and selective detection of Pb2+ [19, 20]. Fe3+ ions are important for most organisms, Therefore, detection of Fe3+ ions for life science is of great significance [21-23]. It is reported that a novel supramolecular organogel (P5N-OG) showing ultrasensitive response for Fe3+ and H2PO4- through the competition between cation–p and exo-wall p–p interactions [24]. Among these different kinds of sensing materials, the selective sensing of supramolecular compounds benefitting from the dynamic and reversible nature of supramolecular interactions, including C@H…π, π…π, cation…π, etc. [16]. The sensitive electrochemical lead ion sensors have good charge-transport capacity, low detection limit and might exist complexity of design. MOFs combine the advantages of the highly regular porous structures, various luminescence mechanisms and diversified host-guest interactions, which make they reflect simple and quick detection, high selectivity, easy design and operation.
Recently, by using chromophores organic ligands or fluorescent metal ions, fluorescence MOFs have been reported [25, 26]. Sun's group have reported serials of 3D porous MOFs, which have showed rapid and selective detection of metal ions and NACs [27, 28]. It is insufficient to be limited to metal ions for constructing fluorescent MOFs, the role of ligand is more essential. Based on this consideration, a new flexible tetracarboxylate ligand (H4TTTA = 2, 2′, 2′', 2′″-(2, 2′, 4, 4′, 6, 6′-hexamethylbiphenyl-3, 3′, 5, 5′-tetrayl) tetrakis(methylene)tetrakis(sulfanediyl)tetrabenzoic acid) and two new rigid tetracarboxylate ligands (H4TB = 3, 3′, 5, 5′-tetra((4- carboxyphenyl)bimesityl, and H4TEB = 3, 3′, 5, 5′-tetra(4-ethynylbenzoic acid)bimesityl) have been designed and synthesized as the assembly organic ligands (Fig. 1). The three new ligands have some specific characteristics: (ⅰ) Four carboxyl groups can bridge metal ions with multiple coordination modes; (ⅱ) The steric hindrance of methyl will trigger rearrangement of adjacent benzene rings [29-31]; (ⅲ) The flexibility conformational of ligands may offer more possibility to construct new topology networks; (ⅳ) The polycyclic aromatic conjugated ligands will induce fluorescent properties of MOFs. Herein, three new complexes [Cd2(TTTA)(DMF)3]·2DMF (1), [Cd2(TB)(H2O)4]· 3DMF·H2O (2) and [Cd(TEB)0.5]·2DMF·4H2O (3) are constructed by the above three ligands and Cd2+ ion. Complex 1 is a 2D 3, 4- connected network with 3, 4L13 topology. Complex 2 is a 3D framework with a 2-fold interpenetrating tfa topology, which exhibits selective adsorption of CO2 over CH4 and displays interesting fluorescent sensing properties for Fe3+ ion and rapid detection of NACs. Complex 3 has a 3D 4-fold interpenetrating 4- connected dia topology.

|
Download:
|
| Fig. 1. The three ligands designed and used in this paper. | |
H4TTTA, H4TB and H4TEB are synthesized by Suzuki and Sonogashira coupling reaction followed by hydrolysis with dilute HCl (Scheme S1 in Supporting information). Detailed synthesizing procedures of the three complexes are depicted in Supporting information.
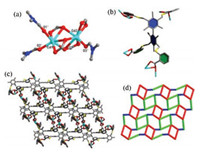
Complex 1 is constructed by using flexible H4TTTA ligand. Single crystal X-ray diffraction analysis reveals that 1 crystallizes in the triclinic p-1 space group. The asymmetrical unit of 1 contains two Cd(Ⅱ) ions, one fully deprotonated TTTA4- ligand and four DMF molecules. Cd1 is bonded to five carboxylate oxygen atoms from three different TTTA4- ligands and the remaining two sites are occupied by oxygen atoms from two different coordinated DMF molecules; Cd2 bridging six oxygen atoms from four different TTTA4- ligands and the residue one oxygen atom from a coordinated DMF molecule (Fig. 2a). H4TTTA ligand is deprotonated during the self-assembly process, the average dihedral angle between the side benzene ring and the adjacent central benzene ring is 75.4° (Fig. 2b). There are two kinds of rectangular macrocycles in this network: one is connected by two half of TTTA4- ligands and two SBUs with the dimensions of 9.2 × 9.4 Å2 (Fig. S1a in Supporting information) and the other is made up of two benzene rings of the rigid center and two SBUs with the dimensions of 15.0 × 7.9 Å2 (Fig. S1b in Supporting information). The metal-macrocycles are further connected one another through the expansion of ligands to generate a 2D layer (Fig. 2c). Topologically, by treating the dimeric Cd SBUs as a 4-connected nodes and the TTTA4- ligands as double Y-shaped linkers, the 2D structure can be classified as 3, 4L13 topology (Fig. 2d).
|
Download:
|
| Fig. 2. (a) Coordination environment of the dimeric Cd2+ ions in 1; (b) Configuration and coordination modes of TTTA4- ligand; (c) and (d) The 2D layer structure and 2D network with 3, 4L13 topology for 1. | |
By using rigid ligand of H4TB and H4TEB, complexes of 2 {[Cd2(TB)(H2O)4]·3DMF·H2O} and 3 {[Cd(TEB)0.5]·2DMF·4H2O} were obtained. Single crystal X-ray diffraction reveals that 2 crystallizes in the triclinic p-1 space group. The asymmetrical unit of 2 contains two Cd(Ⅱ) ions, one fully deprotonated TB4- ligand and four H2O molecules. Cd1 is six-coordinated by three oxygen atoms from three different TB4- ligands and three coordinated water molecules; Cd2 is seven-coordinated by six oxygen atoms from four different TB4- ligands, and one coordinated water molecule (Fig. 3a). All the carboxyl groups of H4TB ligand were deprotonated during the reaction: one adopt bidentate mode to link a Cd2+ ion, one adopt chelating mode to connect a Cd2+ ion, and the remaining two adopt mono-dentate mode to connect four Cd2+ ions (Fig. 3b). Then, the dimeric SBUs are connected by TB4- ligands to generate an open framework with 3D channel which similar pore features in a, b, c axis with the size of 16.0 × 18.7 Å2 (Fig. S2 in Supporting information). Due to the existence of large channel in the single net of 2, two such nets interpenetrate mutually to provide a 3D structure with the channels of 7.5 × 7.8 Å2 (Fig. 3c). Topologically, the H4TB ligands can be simplified as a 4- connected linker and the dimeric SBU is taken as a 4-connected node. Then, the 3D structure can be classified as a classical tfa topology (Fig. 3d).

|
Download:
|
| Fig. 3. (a) Coordination environment of Cd2+ ions in 2; (b) Configuration and coordination modes of organic ligands in 2; (c) The 2-fold interpenetrating 3D framework; (d) The 3D network with tfa topology for 2. | |
Single crystal X-ray diffraction reveals that 3 crystallizes in the tetragonal I4122 space group. The asymmetrical unit of 3 contains one independent Cd(Ⅱ) ion and half fully deprotonated TEB4- ligand. Cd2+ is eight-coordinated by eight oxygen atoms from four different TEB4- ligands (Fig. 4a). One Cd2+ ion is bonded to four TEB4- ligands affording an extended open framework with window of 21.7 × 22.1 Å2 along b axis (Fig. 4b), and four frameworks are interpenetrated with each other to form a rectangular channel with dimensions of 16.5 × 14.0 Å2 (Fig. 4c). Topologically, the Cd2+ ions and H4TEB ligands can be considered as two 4-connected nodes, 3 reveals a typically diamondoid framework (Fig. S3 in Supporting information). The four identical diamondoid networks interpenetrated to giving rise to a 4-fold interpenetrating dia topology (Fig. 4d).

|
Download:
|
| Fig. 4. (a) Configuration and coordination modes of organic ligands in 3; (b) The 1D channel of 3 along b axis; (c) The 4-fold interpenetrating 3D framework; (d) The 3D network with dia topology for 3. | |
As described above, the interpenetration dimensionality significantly influenced the porosity of the final framework, which can be further confirmed by gas-uptake measurements [32]. The N2 sorption isotherms of 2 shows the reversible type Ⅰ, indicating its permanent porosity. The BET surface area calculated from N2 isotherm is 165.8 m2/g, with the pore volume of 0.08 cm3/g (Fig. 5a). The H2 adsorption isotherms at 77 K also exhibits reversible type Ⅰ behavior with the largest adsorption amounts of 28.3 cm3/g (Fig. 5b). In addition, the uptake of CO2 is of 18.2 cm3/g at 273 K, which is much higher than CH4 (5.35 cm3/g at 273 K) (Fig. 5c). It is obvious that 2 exhibits selective adsorption of CO2 over CH4, although the adsorption amounts are somewhat lower than those reported results [33-35]. To further investigate the CO2 selectivity, the CO2/ CH4 selectivity at 273 K is calculated by the ideal adsorbed solution theory (IAST) method. As shown in Fig. 5d, the selectivity has a downward trend in the range of 0–109 kPa. The values of selectivity are all above 9.6, which are comparable with many reported MOFs [36-38]. The selective adsorption may possibly attribute to the combined effects of the pore feature and host-guest interactions.

|
Download:
|
| Fig. 5. (a) and (b) N2 and H2 sorption isotherms for 2 at 77 K. (c) The CO2/CH4 sorption isotherms for 2 at 273 K. (d) The CO2/CH4 selectivity for 2 at 273 K, calculated by the IAST method (CO2/CH4: 50/50). | |
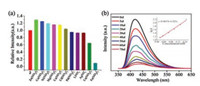
As described above, complex 2 features stable porous 2-fold interpenetrating network, which can be used as a sensor to identify metal ions and NACs through fluorescence alternation [39]. In the measurement, 2.0 mg freshly prepared samples of 2 are ground and suspended in 3.0 mL DMF solution, to which is dropwise added different metal ions in DMF (10.0 mmol/L). We experimented three parallel times and obtained experimental data finally. The fluorescence properties are recorded and listed in Fig. 6a (excitation at 330 nm). Specifically, the fluorescence intensity of 2 at 418 nm decreases slightly when Li+, Ag+, Cd2+, Zn2+, Mg2+, Cu2+ Co2+, Ni2+, Ca2+, Pb2+ ions are added, but decreases significantly when Fe3+ ions are added (Fig. 6b), the quenching efficiency is higher than UPC-19(Zn-MOF) and UPC-20(Zn-MOF) [40]. The color change after adding Fe3+ is shown in Fig. S13 (Supporting information). Furthermore, the detection limit (LOD) is calculated based on the equation: LOD = 3σ/k, σ value is calculated from 11 groups of blank test and K value is based on the linear regression. The calculated detection limit, LOD, is about 0.011 mmol/L for Fe3+, which further confirm the high sensitivity of 2 on the fluorescent detection of Fe3+. Room-temperature emission spectra for complex 2 and free H4TEB ligand are presented in Fig. S5 (Supporting information). The luminescence was generated from H4TEB ligand. In Fe3+ detection, in the view of crystal structures, 2 is microporous and metal ions can enter into the channels, coordination environment of Cd2+ ions in complex 2 are uncoordinated and provide binding sites for metal ions and the relatively small ionic radius of Fe3+ results in easier combination with complex 2 than other metal ion. With the Fe3+ added, complex 2 and Fe3+ is expected to perturb the luminescence of ligand and leads to fluorescence quenching at last.
|
Download:
|
| Fig. 6. (a) Fluorescence intensity of different metal ions in the DMF-emulsion of 2. (b) Effect on the emission spectra of 2 upon incremental addition of Fe3+. Inert: Stern-Volmer plot of I0/I versus the Fe3+ concentration in DMF. | |
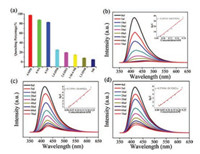
On the other hand, aromatic compounds are potentially neurotoxic and carcinogenic in nature. Rapid and selective detection of benzene compounds, especially for nitroaromatic compounds (NACs) is important [41]. Hence, different benzene compounds in DMF (10 mmol/L) are gradually added into the DMFsuspensions of complex 2 to determine the sensing properties.1, 3-Dinitrobenzene (1, 3-DNB), 2, 4-dinitrotoluene (2, 4-DNT), 1, 4-dinitrobenzene (1, 4-DNB), 4-nitrophenol (4-NP), 4-nitroaniline (4- NA), 1-methyl-4-nitrobenzene (1-M-4-NB), (4-nitrophenyl)-hydrazine (4-NPH) were selected as the investigated reagents. The results show that all NACs can weaken the fluorescence intensity of 2 in suspension, but the quenching percentage show great differences (Fig. 7a). Meanwhile, the fluorescence intensity has relative little change when adding the 1, 3-DNB, 2, 4-DNT, 1, 4-DNB and 1-M-4-NB solution, but the fluorescence intensity produce significant quenching when introducing the 4-NA, 4-NP and 4-NPH solution (Figs. 7b–d). At the same time, the color change after adding NACs is shown in Fig. S14 (Supporting information).
|
Download:
|
| Fig. 7. (a) Percentage of fluorescence quenching, obtained for introducing different NACs into the DMF-emulsion of 2. (b, c and d) Effect on the emission spectra of 2 upon incremental addition of a 4-NPH, 4-NA and 4-NP DMF solution (10 mmol/L). Insert: Stern–Volmer plot of I0/I versus the 4-NPH (b), 4-NA (c) and 4-NP (d). | |
In order to further compare the efficiency of the sensor, the fluorescence quenching efficiency was calculated using the SternVolmer (SV) equation [42], (I0/I) = Ksv [A] + 1, where I0 is the initial fluorescence intensity, I is the fluorescence intensity after adding the analyte, [A] is the molar concentration of analyte [43], and Ksv is the quenching coefficient. According to the equation, the quenching efficiency for the NACs in DMF was found to be the following order: 4-NPH>4-NA>4-NP>>1, 4-DNB>2, 4-DNT>1-M-4- NB>1, 3-DNB > NB (Fig. S16 in Supporting information). The quenching efficiency of 4-NPH is lower than Cd-PDA [44], but higher than [Pr(LOMe)(H2O)4]·2.5DMA·3H2O [45]. The SternVolmer plots are typically linear at low concentrations for 4- NPH, 4-NA and 4-NP. The calculated Ksv are 1.68 × 105, 3.07 × 104 and 2.05 × 104 L/mol (Fig. 7), and the calculated LOD are about 0.004, 0.025, and 0.027 mmol/L, respectively. The results indicate a higher selectivity for 4-NPH, 4-NA and 4-NP, which is possible due to the presence of the NHNH2-, NH2- and OH- group. In NACs detection, due to the strong electron-withdrawing of nitrogen atoms, the competition of absorption of the light source energy and the electronic interaction between the NACs and H4TEB ligands appeared. The highly acidic NH2NH-, NH2- and OH- group can interact strongly with the fluorophore via electrostatic interactions, and the quenching effect can be maintained over a long range due to the energy transfer mechanism. However, because the electron-withdrawing capacity in these functional groups are different: -NHNH2>-NH2>-OH, the quenching efficiency for the NACs in DMF was found to be the following order: 4-NPH>4-NA>4- NP. Thus, the comprehensive reasonwith electrostatic interactions, favorable electrons, and the energy transfer mechanism results in a higher quenching effect.
In conclusion, through design three new methyl-functionality flexible and rigid ligands, three new Cd-based MOFs have been synthesized. Complex 1 is a 2D layer with two rectangular macrocycles and 3 has a 3D 4-connected dia network with a 4-fold interpenetrating, whereas 2 exhibits a 3D 3, 4-connected tfa network with a 2-fold interpenetrating. Complex 2 is porous and exhibits selective adsorption of CO2 over CH4, indicating its potential applications in selective gas separation. Besides that, complex 2 can sense Fe3+ ions in DMF-suspension with a higher selectivity. Meanwhile, 2 shows strong sensing of 4-NPH, 4-NA and 4-NP through fluorescence quenching.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (NSFC, No. 21771191), the Shandong Natural Science Fund (No. ZR2017QB012), the Applied Basic Research Projects of Qingdao (No.16-5-1-95-jch), the Fundamental Research Funds for the Central Universities (Nos.16CX05015A, 18CX06003A, YCX2018071), and the Foundation of State Key Laboratory of Structural Chemistry (No. 20160006).
Appendix A. Supplementary dataSupplementary material related to this article canbefound, in the online version, at doi: https://doi.org/10.1016/j.cclet.2018.12.009.
| [1] |
H.L. Jiang, T.A. Makal, H.C. Zhou, et al., Chem. Rev. 257 (2013) 2232-2249. |
| [2] |
B. Wang, X.L. Lv, D.W. Feng, et al., J. Am. Chem. Soc. 138 (2016) 6204-6216. DOI:10.1021/jacs.6b01663 |
| [3] |
C.Y. Sun, X.L. Wang, C. Qin, et al., Chem.-Eur. J. 19 (2013) 3639-3645. DOI:10.1002/chem.v19.11 |
| [4] |
X.L. Yang, X.H. Chen, G.H. Hou, et al., Adv. Funct. Mater. 26 (2016) 393-398. DOI:10.1002/adfm.v26.3 |
| [5] |
B.Y. Li, Z.J. Zhang, Y. Li, et al., Angew. Chem. Int. Ed. 51 (2012) 1412-1415. DOI:10.1002/anie.201105966 |
| [6] |
M.X. Zhang, C. Chen, Q. Wang, et al., J. Mater. Chem. A 5 (2017) 349-354. DOI:10.1039/C6TA06037D |
| [7] |
J.S. Qin, S.R. Zhang, D.Y. Du, et al., Chem.-Eur. J. 20 (2014) 5625-5630. DOI:10.1002/chem.201304480 |
| [8] |
F.N. Dai, J.M. Dou, H.Y, et al., Inorg. Chem. 49 (2010) 4117-4124. DOI:10.1021/ic902178c |
| [9] |
H.C. Kim, S. Huh, J.Y. Kim, et al., CrystEngComm. 19 (2017) 99-109. DOI:10.1039/C6CE02122K |
| [10] |
L.B. Sun, H.Z. Xing, Z.Q. Liang, et al., Chem. Commun. 49 (2013) 11155-11157. DOI:10.1039/c3cc43383h |
| [11] |
K. Koh, A.G. Wong-Foy, A.J. Matzger, J. Am. Chem. Soc. 132 (2010) 15005-15010. DOI:10.1021/ja1065009 |
| [12] |
X.J. Wang, P.Z. Li, L. Liu, et al., Chem. Commun. 48 (2012) 10286-10288. DOI:10.1039/c2cc34921c |
| [13] |
P.Z. Li, Y.L. Zhao, Chem.-Asian J. 8 (2013) 1680-1691. DOI:10.1002/asia.v8.8 |
| [14] |
Q. Lin, K.P. Zhong, Y.M. Zhang, et al., Macromolecules 50 (2017) 7863-7871. DOI:10.1021/acs.macromol.7b01835 |
| [15] |
Q. Lin, Y.Q. Fan, T.B. Wei, et al., ACS Sustainable Chem. Eng. 6 (2018) 8775-8781. DOI:10.1021/acssuschemeng.8b01124 |
| [16] |
Q. Lin, Y.Q. Fan, T.B. Wei, et al., Chem.-Eur. J. 24 (2018) 777-783. DOI:10.1002/chem.201705107 |
| [17] |
Q. Lin, P.P. Mao, T.B. Wei, et al., Soft Matter 13 (2017) 7085-7089. DOI:10.1039/C7SM01447C |
| [18] |
Q. Lin, X.M. Jiang, T.B. Wei, et al., Sens. or Actuat. B-Chem. 272 (2018) 139-145. |
| [19] |
G.M. Zeng, Y. Zhu, Y. Zhang, et al., Environ. Sci. Nano 3 (2016) 1504-1509. DOI:10.1039/C6EN00323K |
| [20] |
Y. Zhu, G.M. Zeng, Y. Zhang, et al., Analyst 139 (2014) 5014-5020. DOI:10.1039/C4AN00874J |
| [21] |
M. Zheng, H.Q. Tan, Z.G. Xie, et al., Appl. Mater. Interfaces 5 (2013) 1078-1083. DOI:10.1021/am302862k |
| [22] |
W. Wang, J. Yang, D.F. Sun, et al., Cryst. Growth Des. 15 (2015) 2589-2592. DOI:10.1021/acs.cgd.5b00381 |
| [23] |
R.M. Wang, X.B. Liu, D.F. Sun, et al., Inorg. Chem. 55 (2016) 1782-1787. DOI:10.1021/acs.inorgchem.5b02693 |
| [24] |
Y.M. Zhang, W. Zhu, Q. Lin, et al., Chem. Commun. 54 (2018) 4549-4552. DOI:10.1039/C8CC00814K |
| [25] |
Y.J. Cui, Y.F. Yue, G.D. Qian, et al., Chem. Rev. 112 (2012) 1126-1162. DOI:10.1021/cr200101d |
| [26] |
L.E. Kreno, K. Leong, O.K. Farha, et al., Chem. Rev. 112 (2012) 1105-1125. DOI:10.1021/cr200324t |
| [27] |
M. Guo, Z.M. Sun, J. Mater. Chem. 22 (2012) 15939-15946. DOI:10.1039/c2jm32066e |
| [28] |
P.Z. Li, X. Lu, B. Liu, et al., Inorg. Chem. 46 (2007) 5823-5825. DOI:10.1021/ic070214i |
| [29] |
D. Tian, Q. Chen, Y. Li, et al., Angew. Chem. Int. Ed. 126 (2014) 856-860. DOI:10.1002/ange.201307681 |
| [30] |
Q.Y. Yang, D.H. Liu, J.R. Li, et al., Chem. Rev. 113 (2013) 8261-8323. DOI:10.1021/cr400005f |
| [31] |
W.G. Lu, Z.W. Wei, Z.Y. Gu, et al., Chem. Soc. Rev. 43 (2014) 5561-5593. DOI:10.1039/C4CS00003J |
| [32] |
W.D. Fan, H. Lin, R.M. Wang, et al., Inorg. Chem. 55 (2016) 6420-6425. DOI:10.1021/acs.inorgchem.6b00278 |
| [33] |
Z.H. Xuan, D.S. Zhang, Z. Chang, et al., Inorg. Chem. 53 (2014) 8985-8990. DOI:10.1021/ic500905z |
| [34] |
B. Wang, H. Huang, X.L. Lv, et al., Inorg. Chem. 53 (2014) 9254-9259. DOI:10.1021/ic5013473 |
| [35] |
Y.Q. Chen, Y.K. Qu, G.R. Li, et al., Inorg. Chem. 53 (2014) 8842-8844. DOI:10.1021/ic500788z |
| [36] |
P. Xydias, I. Spanopoulos, E. Klontzas, et al., Inorg. Chem. 53 (2014) 679-681. DOI:10.1021/ic402430n |
| [37] |
Q. Yang, A.D. Wiersum, P.L. Llewellyn, et al., Chem. Commun. 47 (2011) 9603-9605. DOI:10.1039/c1cc13543k |
| [38] |
W. Zhang, H. Huang, C. Zhong, et al., Phys. Chem. 14 (2012) 2317-2325. |
| [39] |
S. Pramanik, C. Zheng, X. Zhang, et al., J. Am. Chem. Soc. 133 (2011) 4153-4155. DOI:10.1021/ja106851d |
| [40] |
X.Z. Song, S.Y. Song, S.N. Zhao, et al., Adv. Funct. Mater. 24 (2014) 4034-4041. DOI:10.1002/adfm.201303986 |
| [41] |
A. Rose, Z. Zhu, C.F. Madigan, et al., Nature 434 (2005) 876-879. DOI:10.1038/nature03438 |
| [42] |
A. Ganguly, B.K. Paul, S. Ghosh, et al., Analyst 138 (2013) 6532-6541. DOI:10.1039/c3an00155e |
| [43] |
P.Y. Wu, Y.H. Liu, Y. Li, et al., J. Mater. Chem. A 4 (2016) 16349-16355. DOI:10.1039/C6TA06997E |
| [44] |
X.B. Liu, H. Lin, D.F. Sun, et al., Dalton Trans. 45 (2016) 3743-3749. DOI:10.1039/C5DT04339E |
| [45] |
S.S. Nagarkar, B. Joarder, A.K. Chaudhari, et al., Angew. Chem. Int. Ed. 52 (2013) 2881-2885. DOI:10.1002/anie.201208885 |
 2019, Vol. 30
2019, Vol. 30 

