b Guangxi Key Laboratory of Information Materials, Guilin University of Electronic Technology (GUET), Guilin 541004, China;
c Shanghai Innovation Institute for Materials, Shanghai 200444, China
Antibiotics are widely used in human and livestock to improve growth rate and disease prevention. Norfloxacin (NOR) is a kind of common antibiotic with wide spectrum of antibacterials and few side effects. However, due to the relatively weak metabolism and absorption, a large proportion of the given NOR will be discharged into the environment with the domestic waste water or feces. If the residue is not removed in time, they will accumulate in the water and cause a series of ecological problems, which will pose a serious threat to the microbial population and the health of human and livestock [1-5]. In recent years, with the improvement of living standards, people pay more and more attention to the ecological environment. Therefore, the removal of the residual antibiotics in the aquatic environment has also attracted widespread attentions. Photocatalytic technology is green and efficient, and can effectively degrade organic pollutants into CO2, H2O and other small molecules at room temperature. As a typical Aurivillius multicomponent oxide possessing a layered structure, Bi2WO6 has attracted great attentions because of the excellent intrinsic physicochemical properties and structure-property relationships [6-9]. It has been discovered that Bi2WO6 possesses photocatalytic activity [10, 11], besides ferroelectricity, piezoelectricity and nonlinear dielectric susceptibility [12]. For example, Kudo et al. [10] found that Bi2WO6 exhibited excellent photocatalytic activities for O2 evolution. Tang et al. [11] revealed that Bi2WO6 could catalytic degrade organic compounds under visible-light irradiation. Kaur et al. [13] reported that Bi2WO6 showed good photocatalytic activity for levofloxacin degradation. So, photocatalytic technology has been recognized as one of the most ideal and promising models for solving antibiotic residues in the aqueous problems [14]. Recently, the photodegradation of norfloxacin, enrofloxacin and ciprofloxacin in the aquatic environments has been investigated [15]. However, to the best of our knowledge, there is no investigations have been made about the photocatalytic degradation of NOR in the presence of 3D navel-like BWO hierarchical microspheres(HMSs) under light irradiation.
Herein, 3D navel-like BWO HMSs have been successfully prepared with hydrothermal method. The complex structures with large specific surface area and high crystallinity have advantages of efficient light utilization, enhanced dye absorption and fast separation/transfer of photoexcited electrons (e-) and holes (h+). The structure, morphology, and optical properties of the as-prepared samples were investigated by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), Brunauer-Emmett-Teller analysis (BET), and UV–vis spectrophotometer. The photocatalytic activity of the BWO samples were studied by measuring the degradation on NOR under UV light irradiation (λ = 365 nm). The experiments are similar to that described in our previous paper [16-19].
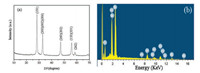
The typical XRD pattern of the as-obtained BWO samples was showed in Fig. 1a. The BWO powders displayed a good crystallinity. All the peaks can be assigned to the orthorhombic phase Bi2WO6, which is in good agreement with the reported data (JCPDS card No. 39-0256). No other impurities were detected, indicating that the obtained samples have a high purity. High crystallinity generally means fewer traps and stronger photocatalytic activity. The energy dispersive X-ray spectrometer (EDS) study (Fig. 1b) reveals that the resulting sample contained only three elements, namely Bi, W and O. The atomic ratio of Bi to W is 2:1.10, which is close to stoichiometric proportion of Bi2WO6.
|
Download:
|
| Fig. 1. XRD (a) and EDS (b) patterns of the BWO samples. | |
Fig. 2a is the typical SEM image of the obtained BWO samples, revealing that the samples consist of a large quantity of microspheres. The magnified image (Fig. 2b) shows that the microspheres have a navel-like cavity and exhibit a hierarchical structure with an average diameter of 2 μm. A magnified SEM image of an individual microsphere indicates that the BWO HMSs are built from several dozen of nanosheets, as shown in Fig. 2c. These nanosheets with a thickness of about 30 nm and a width of 1 μm to 1.5 μm are sequentially stacked together to form navel-like hierarchical structures. Furthermore, it is worth noting that although these nanosheets are not highly closepacked, all of the navel-like hierarchical structures maintained their integrity after vigorous ultrasonication for 30 min, indicating that the resulting samples have a good structural stability. Fig. 2d presents the TEM image of an individual microsphere. It confirms that the diameter of the obtained BWO microsphere is about 2 μm, which is in accordance with the SEM images. A selected-area electron diffraction (SAED) pattern of the microsphere was also recorded (inset of Fig. 2d). It is clear that obtained BWO is polycrystalline in nature. Fig. 2e shows a typical TEM image of a quarter of an individual BWO microsphere, which indicates that the basic BWO nanosheets are sequentially stacked and the thickness is about 30 nm. Fig. 2f is the HRTEM image taken from the edge of the microsphere. As can be seen from the HRTEM image, a group of clear parallel crystal planes with the interspacing of 0.272 nm, corresponding to the (200) plane of orthorhombic Bi2WO6.

|
Download:
|
| Fig. 2. SEM and TEM images of the as-synthesized BWO samples. (a) panoramic SEM image, (b) magnified SEM image, (c) SEM image of an individual hierarchical microsphere, (d) TEM image of an individual microsphere and inserted SAED pattern, (e) TEM image of a quarter of the BWO microsphere, (f) HRTEM image taken from the edge of the microsphere. | |
The specific surface areas were measured according to the Brunauer-Eμmett-Teller (BET) analysis. The nitrogen adsorptiondesorption isotherm of the obtained BWO samples was shown in Fig. S1 (Supporting information). These isotherms can be categorized as type Ⅳ with a distinct hysteresis loop observed in the range of 0.5–1.0 P/P0, which implies the presence of mesopores. Moreover, it can be seen that the hysteresis loop shifts approach P/P0 = 1, indicating the existence of macropores [20, 21]. The smaller mesopores may arise from the crystal growth process, whereas the larger macropores can be attributed to the space between the intercrossed BWO nanosheets. The BET surface area is about 35.40 m2/g, which is larger than that of the reported [22]. It is apparent that those BWO hierarchical microspheres constructed from relatively small nanosheets have a larger specific surface area. It is generally accepted that the large surface area helps to increase the reaction sites of catalytic reaction and improve the gas sensing properties of the materials [23-26].
PL emission spectra of the as-synthesized BWO and the reported [27] under excitation at 280 nm were given in Fig. S2 (Supporting information). It is found that the BWO exhibited much lower emission intensity than the reported, indicating that the recombination of e--h+ pairs is greatly suppressed during photocatalysis. The effective separation of the photogenerated e- and h+ in the BWO samples is also responsible for the high photocatalytic activity.
The UV–vis diffuse reflectance spectrum of the BWO samples was showed in Fig. 3a, which revealed that the BWO samples have an obvious blue shift in the absorption edge compared with the reported [28]. For BWO, a direct band gap semiconductor materials, the band gap energy (Eg) can be evaluated by the following formula: (αhν)2 = B(hν-Eg) [29]. The (αhν)2 vs. hν curve for the BWO samples was showed in Fig. 3a (inset). The Eg value of the BWO samples is 3.3 eV, which is a little different from the reported [28]. The above results indicated that the novel hierarchical structures led to the difference in absorption edge shift and band gap energy [30]. The big Eg value implies the possibility of high photocatalytic activity of this kind of material under UV light irradiation. Fig. 3b showed the UV–vis spectra of the NOR during the degradation process at different time intervals. It could be clearly seen that the intensity of the characteristic absorption peak (λ = 286 nm) of the NOR significantly decreases and about 67% NOR was degraded in 8 h. In addition, the position of the characteristic absorption peak shifted slightly to a short wavelength. The significant decrease and shift of the characteristic absorption peak indicate that the BWO sample exhibits excellent photocatalytic activity in the degradation of NOR. The photodegradation curve of NOR and the plot of ln(C/C0) against time were shown in Fig. S3 (Supporting information). The obtained BWO samples showed decent photocatalytic activity with a rate constant k of 0.14047 h-1. The excellent photocatalytic properties may be attributed to the high specific surface area, hierarchical structures, big Eg value, and high crystallinity of the obtained BWO samples.

|
Download:
|
| Fig. 3. (a) UV–vis diffuse reflectance. Inset: the (αhν)2-hν curve for the samples. (b) Absorption spectra of a solution of NOR in the presence of the 3D navel-like BWO hierarchical microspheres. | |
In summary, 3D navel-like Bi2WO6 (BWO) hierarchical microspheres (HMSs) with high crystallinity were successfully prepared using hydrothermal method. UV–vis spectrum revealed that the band gap energy of the obtained BWO samples was about 3.3 eV. The specific surface area of the BWO samples was about 35.40 m2/g. The 3D navel-like BWO hierarchical structure composed of well-ordered nanosheets displayed the excellent photocatalytic activity, and the degradation rate of NOR was about 67%. We believe these obtained BWO HMSs will offer new opportunities for the development of electronics, optics, catalytic, and gas sensing applications.
AcknowledgmentsThis research was jointly sponsored by the Shanghai Municipal Natural Science Foundation (No. 18ZR1415700), Guangxi Key Laboratory of Information Materials (GUET, No. 171011-K), Leap Project and Postgraduate fund (SSPU, Nos. EGD18XQD26, EGD18YJ0049, EGD17YJ0005), the key subject of SSPU (No. 4: Material Science and Engineering, XXKZD1601), and Gaoyuan Discipline of Shanghai–Environmental Science and Engineering (Resource Recycling Science and Engineering).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2018.10.036.
| [1] |
M.H. Khan, H. Bae, J.Y. Jung, J. Hazard. Mater. 181 (2010) 659-665. DOI:10.1016/j.jhazmat.2010.05.063 |
| [2] |
Z.Y. Lu, X.X. Zhao, Z. Zhu, et al., Chem.-Eur. J. 21 (2015) 18528-18533. DOI:10.1002/chem.201503759 |
| [3] |
Y. Junejo, A. Güner, A. Baykal, et al., Appl. Surf. Sci. 317 (2014) 914-922. DOI:10.1016/j.apsusc.2014.08.133 |
| [4] |
X.L. Guo, D. Li, J. Wan, X. Yu, Electrochim. Acta 180 (2015) 957-964. DOI:10.1016/j.electacta.2015.09.055 |
| [5] |
C. Cai, H.L. Liu, B. Wang, J. Hazard. Mater. 331 (2017) 265-272. DOI:10.1016/j.jhazmat.2017.02.034 |
| [6] |
G. Zhang, Z.Y. Hu, M. Sun, et al., Adv. Funct. Mater. 25 (2015) 3726-3734. DOI:10.1002/adfm.v25.24 |
| [7] |
M. Chen, W. Chu, Appl. Catal. B 168- 169 (2015) 175-182. |
| [8] |
L. Liang, Y. Tursun, A. Nulahong, et al., Ultrason. Sonochem. 39 (2017) 93-100. DOI:10.1016/j.ultsonch.2017.03.054 |
| [9] |
R. He, S. Cao, J.G. Yu, Acta Phys. Chim. Sin. 32 (2016) 2841-2870. |
| [10] |
A. Kudo, S. Hijii, Chem. Lett. 10 (1999) 1103-1104. |
| [11] |
J.W. Tang, Z.G. Zou, J.H. Ye, Catal. Lett. 92 (2004) 53-56. DOI:10.1023/B:CATL.0000011086.20412.aa |
| [12] |
A. Feteira, D.C. Sinclair, J. Am. Ceram. Soc. 91 (2008) 1338-1341. DOI:10.1111/j.1551-2916.2008.02272.x |
| [13] |
A. Kaur, S.K. Kansal, Chem. Eng. 302 (2016) 194-203. DOI:10.1016/j.cej.2016.05.010 |
| [14] |
Y. Liang, S. Lin, L. Liu, et al., Appl. Catal. B 164 (2015) 192-203. DOI:10.1016/j.apcatb.2014.08.048 |
| [15] |
S. Babić, M. Periša, I. Škorić, Chemosphere 91 (2013) 1635-1642. DOI:10.1016/j.chemosphere.2012.12.072 |
| [16] |
L.P. Zhu, L.L. Wang, N.C. Bing, et al., ACS Appl. Mater. Interfaces 5 (2013) 12478-12487. DOI:10.1021/am403720r |
| [17] |
L.P. Zhu, N.C. Bing, L.L. Wang, et al., Dalton Trans. 41 (2012) 2959-2965. DOI:10.1039/c2dt11822j |
| [18] |
L.P. Zhu, G.H. Liao, N.C. Bing, et al., CrystEngComm 12 (2010) 3791-3796. DOI:10.1039/c0ce00038h |
| [19] |
L.P. Zhu, C. Huang, J.W. Liu, et al., Mater. Lett. 106 (2013) 348-351. DOI:10.1016/j.matlet.2013.05.093 |
| [20] |
D.V. Bavykin, V.N. Parmon, A.A. Lapkin, et al., J. Mater. Chem. 14 (2004) 3370-3377. DOI:10.1039/b406378c |
| [21] |
J. Yu, G. Yu, B. Cheng, J. Mol. Catal. A 249 (2006) 135-142. DOI:10.1016/j.molcata.2006.01.003 |
| [22] |
D. Ma, S.M. Huang, J. Phys. Chem. C 113 (2009) 4369-4374. DOI:10.1021/jp810726d |
| [23] |
J. Tang, Z. Zou, J. Ye, Chem. Mater. 16 (2004) 1644-1649. DOI:10.1021/cm0353815 |
| [24] |
W. Luo, T. Zhao, Y. Li, et al., J. Am. Chem. Soc. 138 (2016) 12586-12595. DOI:10.1021/jacs.6b07355 |
| [25] |
W. Luo, Y. Li, J. Dong, et al., Angew. Chem. 125 (2013) 10699-10704. DOI:10.1002/ange.201303353 |
| [26] |
T. Zhao, W. Luo, Y. Deng, et al., Nano Energy 26 (2016) 16-25. DOI:10.1016/j.nanoen.2016.04.050 |
| [27] |
L. Xiang, L. Chen, C.H. Mo, et al., J. Mater. Sci. 53 (2018) 7657-7671. |
| [28] |
Y. Zhao, Y. Wang, E. Liu, J. Fan, X. Hu, Appl. Surf. Sci. 436 (2018) 854-864. DOI:10.1016/j.apsusc.2017.12.064 |
| [29] |
M.A. Butler, J. Appl. Phys. 48 (1977) 1914-1918. DOI:10.1063/1.323948 |
| [30] |
Q. Zhu, X. Shen, L. Wang, et al., Chin. Chem. Lett. 29 (2018) 1310-1312. DOI:10.1016/j.cclet.2017.12.018 |
 2019, Vol. 30
2019, Vol. 30 

