b Key Laboratory of Synthetic and Self-Assembly Chemistry for Organic Functional Molecules, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
Environmental pollution due to science and technological development is one of the most serious problems of this century. Lead (Pb) and cadmium (Cd) are two common pollutants introduced into natural waters from a variety of industrial effluent. They are also the most toxic heavy metal elements to humans and animals, even at their low concentrations [1]. For instance, Pb(Ⅱ) can cause mental deficiency, brain damage, anemia as well as behavioral problems [2]. In addition, Cd(Ⅱ) in humans can cause serious damage to kidney and bones. Therefore, it is an urgent task to remove these two toxic heavy metals from the polluted wastewaters.
Several physicochemical methods have been used for the removal of toxic metals, in which adsorption methodology is considered to be high efficiency, cost-effectiveness, simple operation, and environmental friendliness [3]. However, development of new materials with highly heavy metal-removal capacity is still needed for practical application. Chitosan has been reported to be a biotype adsorbent due to its advantages about non-toxicity, biodegradability and biocompatibility [4]. It is used for metal ion concentrating and removal because the large number of amino and hydroxyl functional groups on its molecules can chelate metal ions [5-7]. Chitosan can be processed into different shapes. The nanosize chitosan has higher adsorption capacity for both Pb(Ⅱ) and Cd (Ⅱ) compared with chitosan flakes or chitosan granules. The chitosan nanofibers prepared by electrospinning process, due to the high specific area and high porosity, can be used in the removal of heavy metal ions [8]. The electrospun chitosan nanofibers could adsorb 263.2 mg/g Pb(Ⅱ), which were much higher than those of chitosan flakes (12.6 mg/g) and chitosan granules (100.1 mg/g) [9, 10]. Meanwhile, the maximum capacities of Cd(Ⅱ) adsorbed by plain chitosan flakes and chitosan granules in batch studies were found to be 85.5 mg/g [11] and 53.4 mg/g [12], respectively, which was much lower than that of the electrospun chitosan nanofibers (143.8 mg/g) [13].
Crosslinking is necessary for chitosan due to its instability in water pollutants. However, the adsorption capacity of crosslinked chitosan for heavy metal ions was reported often much lower than that of raw chitosan due to the decreased adsorption sites. Ionimprinting method is an alternative to compensate excessively crosslinking. Ion-imprinted polymer has gained much attention in generating recognition sites by template ions on crosslinked polymer matrixes. Some interest has been focused on the preparation of various Pb(Ⅱ)-imprinted or Cd(Ⅱ)-imprinted chitosan beads or hydrogels to improve the adsorption capacity for the imprinted metals. For instance, Liu prepared Cd(Ⅱ)-imprinted chitosan resin (Cd-ICR) and used as an adsorbent for selective removal of Cd(Ⅱ) from aqueous solutions. The maximum adsorption capacity of Cd-ICR for Cd(Ⅱ) was 89.0 mg/g [14]. Lu reported an improved synthesis method of preparation of Pb(Ⅱ)- imprinted chitosan (Pb(Ⅱ)-CS) bead and the adsorption capacity of Pb(Ⅱ)-CS bead reached 79.2 mg/g for Pb(Ⅱ) [15]. However, only few investigations on the adsorption capacity of the ion-imprinted chitosan electrospun nanofibers have been reported. In our previous studies, the Pb-imprinted and Cd-imprinted chitosan electrospun nanofibers were prepared and the adsorption capacity could arrived at 577 mg/g for Pb(Ⅱ) [16] and 364 mg/g for Cd(Ⅱ) [17], respectively.
Nowadays, imprinting two or more targets (molecules or ions) in a polymer format is an upcoming technology. Dickert [18] introduced the idea of "double molecular imprinting" using anthracene and chrysene as templates that were simultaneously imprinted in a crosslinked polyurethane thin film, which was able to recognize the both templates. Compared with single template imprinted polymers, hierarchically imprinted polymers and double-template imprinted polymers demonstrate high binding capacity, high selectivity, and fast mass transfer [19, 20]. For example, Birlik [21] prepared double-imprinted polymers in which modified chitosan was complexed with copper(Ⅱ) ions and then reacted with 3-(2-aminoethylamino) propyltrimethoxysilane (AAPTS). The double-imprinted polymers could be used many times without decreasing their adsorption capacities significantly. Wei [22] prepared a novel magnetic ion-imprinted polymer (MIIP) by inverse microemulsion polymerization using ethylene glycol dimethacrylate (EGDMA) as the cross-linker, and 2, 2-azo-bisisobutyronitrile (AIBN) as the initiator. Fe3O4 particles were incorporated into the imprinted polymer matrix containing 4- vinyl pyridine and acrylate-modified Spirulina platensis as the functional monomers, Pb(Ⅱ) and Cd(Ⅱ) as double-templates. The maximum static adsorption capacities of MIIP for Pb(Ⅱ) and Cd(Ⅱ) were 108 mg/g and 56 mg/g, respectively.
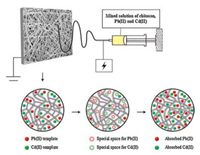
This work deals with the removal of Pb(Ⅱ) and Cd(Ⅱ) in multiform systems using Pb(Ⅱ)-Cd(Ⅱ) double-imprinted electrospun cross-linked chitosan nanofibers (Pd/Cd-DIECCNs). To our knowledge, there was only single-imprinted chitosan electrospun nanofibers system being investigated and no report on the preparation and adsorption properties of the Pd/Cd-DIECCNs. In this study, Pd/Cd-DIECCNs were prepared by using the electrospun chitosan nanofibers as matrix and glutaradehyde as a crosslinker. The double-ion imprinting method was applied on the electrospun chitosan nanofibers with both Pb(Ⅱ) and Cd(Ⅱ) as templates. The effects of pH, amount of adsorbent, contact time, temperature on the adsorption capacity for Pb(Ⅱ) or Cd(Ⅱ) were investigated to explore the possibility of the Pd/Cd-DIECCNs in industrial applications. Pd/Cd-DIECCN is a superior sorbent for removal of both Pb(Ⅱ) and Cd(Ⅱ) from aqueous solution, which will be useful for further applications in the treatment of practical waste effluents.
The preparation route of the chitosan adsorbent is outlined in Scheme 1. The detailed preparation and characterization sections of Pd/Cd-DIECCNs can be found in Supporting information.
|
Download:
|
| Scheme 1. Preparation process of the Pd/Cd-DIECCNs. | |
In electrospinng process, it is very important to produce the nanofibers with minimum diameter, because the thinner and homogeneous fibers provide maximum surface area and porosity, which results in higher efficient adsorbents [23]. In our previous study, the minimum fiber average diameter of the Pd/Cd-DIECCNs was found to be about 110 nm [24], as shown in Fig. 1a. Meanwhile, the Pd/Cd-DIECCNs had good solution stability and kept good nanofiber morphology (Fig. 1b) after immersing in the mixed solutions for 24 h, which can be used as adsorbent for further adsorption investigation.

|
Download:
|
| Fig. 1. SEM images of the Pd/Cd-DIECCNs: (a) before adsorption; (b) after adsorption. | |
The capacities of the Pd/Cd-DIECCNs for Pb(Ⅱ) and Cd(Ⅱ) were investigated in their mixed solutions with different metal ions ratios and the results were shown in Table 1. The Pd/Cd-DIECCNs showed good adsorption capacities either for Pb(Ⅱ) or for Cd(Ⅱ) in their mixed solutions. When the ratio between Pb(Ⅱ) and Cd(Ⅱ) in the mixed solutions was 2:1, the total adsorption capacities reached 908 mg/g, i.e., 567 mg/g for Pb(Ⅱ) and 341 mg/g for Cd(Ⅱ). The ratio was just accordant with that of the double-imprinted ions in the Pd/Cd-DIECCNs. It clearly indicates that the Pd/Cd-DIECCNs developed in this study are far higher than those chitosan adsorbents reported in adsorption capacities. The reasons may be that the Pd/Cd-DIECCNs have more adsorption sites for Pb(Ⅱ) and Cd(Ⅱ) due to using the imprinting technology on the one hand. And the nanofiber structure of the Pd/Cd-DIECCNs prefers adsorption on the other hand.
|
|
Table 1 Adsorption capacities of the Pd/Cd-DIECCNs with different ratios of Pb(Ⅱ) and Cd(Ⅱ) in the mixed solutions. |
The pH of the medium is an important controlling parameter in adsorption process. The binding of metal ions with the surface functional groups of the adsorbent is strongly pH dependent. To inhibit the precipitation of lead hydroxide and cadmium hydroxide under alkaline conditions, all adsorption experiments were carried out in low acidic medium at pH < 7.0. The effect of pH on Pb(Ⅱ) and Cd(Ⅱ) uptakes of the Pd/Cd-DIECCNs was investigated and the results were shown in Fig. 2. The adsorption capacity of the Pd/CdDIECCNs for Pb(Ⅱ) or Cd(Ⅱ) increased with the increase of solution pH values from 2.0 to 6.0 and attained the maximum values of 567 mg/g (for Pb(Ⅱ)) and 341 mg/g (for Cd(Ⅱ)) at pH 6.0, respectively. The low uptake of Pb(Ⅱ) or Cd(Ⅱ) ions at lower pH was attributed to the high concentration of hydrogen ions. Most amine groups of the Pd/Cd-DIECCNs became protonated (-NH3+) at lower pH values; this increased the electrostatic repulsion between Pb(Ⅱ) or Cd(Ⅱ) and the adsorbents, resulting in the reduction of the Pb(Ⅱ) and Cd(Ⅱ) adsorption capacity, indicating that hydrogen ions were preferentially adsorbed [25]. When the pH was increased from 2.0 to 6.0, the amino groups became deprotonated; hence, there were enough binding sites to bind with Pb(Ⅱ) and Cd(Ⅱ). Therefore, pH 6.0 was selected as the optimal value for Pb(Ⅱ) and Cd(Ⅱ) adsorption on the Pd/Cd-DIECCNs.

|
Download:
|
| Fig. 2. Pb(Ⅱ) and Cd(Ⅱ) adsorption capacities of the Pd/Cd- DIECCNs at different pH values. | |
Effect of contact time on the Pb(Ⅱ) and Cd(Ⅱ) adsorption capacities by the Pd/Cd-DIECCNs was shown in Fig. 3. The slope of the lines combining the data points in the figure reflected the adsorption rates. As can be seen from Fig. 3, both the Pb(Ⅱ) and Cd(Ⅱ) ions adsorption capacity increased abruptly with the adsorption time increasing, and then maximum values were reached within 8 h. In the beginning of fast adsorption, it may be explained due to the availability of more number of adsorption sites. After the initial adsorption, the available sites in the adsorbent reduced and thus rate of adsorption further decreased [14], which attained a limiting value at equilibrium. The maximum adsorption capacity of the Pd/Cd-DIECCNs was about 567 mg/g (for Pb(Ⅱ)) and 341 mg/g (for Cd(Ⅱ)), respectively. Due to the preference of short adsorption time for the minimum energy consumption, 8 h was chosen as the equilibrium adsorption time in the following adsorption experiments.

|
Download:
|
| Fig. 3. Relationship between contact time and adsorption capacities of the Pd/CdDIECCNs for Pb(Ⅱ) and Cd(Ⅱ). | |
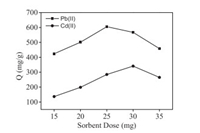
Fig. 4 displayed the effect of the Pd/Cd-DIECCNs dose on the Pb(Ⅱ) and Cd(Ⅱ) adsorption capacity in the mixed solutions. With increasing the dose of the Pd/Cd-DIECCNs, both the Pb(Ⅱ) and Cd(Ⅱ) adsorption capacity increased and trended to reach a maximum value. However, further increase of the Pd/Cd-DIECCNs dose resulted in the decrease of peak adsorption capacity. This suggests that after a certain dose of adsorbent, the amount of free ions changes only slightly even with further addition of the dose of adsorbents. The maximum adsorption capacity for Pb(Ⅱ) was 605 mg/g using 25 mg of the Pd/Cd-DIECCNs. Meanwhile, the maximum adsorption capacity for Cd(Ⅱ) was 341 mg/g with 30 mg of the Pd/Cd-DIECCNs. However, the maximum sum value of adsorption capacities of Pb(Ⅱ) and Cd(Ⅱ) attained 908 mg/g when the Pd/Cd-DIECCNs dose was 30 mg. Therefore, the optimum adsorbent dose was set at 30 mg due to the maximum total adsorption capacity.
|
Download:
|
| Fig. 4. Relationship between the Pd/Cd-DIECCNs dose and adsorption capacities for Pb(Ⅱ) and Cd(Ⅱ). | |
The adsorption capacity of the Pd/Cd-DIECCNs for Pb(Ⅱ) and Cd(Ⅱ) in the mixed solutions increased as the temperature was raised from 15 ℃ to 30 ℃, as shown in Fig. 5. It is apparent that at the higher temperature (< 30 ℃), the adsorption of Pb(Ⅱ) and Cd(Ⅱ) onto the Pd/Cd-DIECCNs or the formation of Pb(Ⅱ) and Cd(Ⅱ) complexes was favored. With the increase of temperature, the speed and range of motion of Pb(Ⅱ) and Cd(Ⅱ) ions became faster and wider, which increased the chance of adsorption. However, when the temperature exceeded 30 ℃, a higher temperature favored desorption or dechelation, which indicated that 30 ℃ was the optimal temperature for the adsorption of Pb(Ⅱ) and Cd(Ⅱ) onto the Pd/Cd-DIECCNs.

|
Download:
|
| Fig. 5. Relationship between temperature and adsorption capacities for Pb(Ⅱ) and Cd(Ⅱ) of the Pd/Cd-DIECCNs. | |
One of the difficulties in studying the adsorption of metal ions from wastewaters is the presence of a multitude of metals. When in the aquatic system there are more of one metal, may occur a competition between metals. So that, the evaluation, interpretation and representation of results are more complex than in single systems, and usually, can occur several types of responses [26-31]. Equilibrium models for multimetallic systems attempt to provide the relationship between the retained amount of one component and the concentration of other components, whether they remain in solution as if they have been retained. In order to characterize the adsorption behavior of dual ions, Extended Langmuir isotherm was adopted to investigate the adsorption process. Extended Langmuir isotherm assumes that solid surface is uniform and all ions present in solution compete by the same binding superficial sites. The Extended Langmuir isotherm can be represented by the following equation [29]:
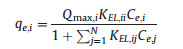
|
where values of Qmax, i, KEL, ii, KEL, ij are obtained by fitting of equation with experimental data for a multimetallic system. For a binary system, the above equation is broken down into the following two:
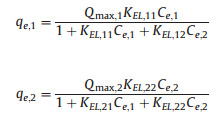
|
In this section, the adsorption experiments were investigated at different concentrations of Pb(Ⅱ) and Cd(Ⅱ). The adsorption isotherm data of the Pd/Cd-DIECCNs were analyzed using the Extended Langmuir isotherm model and the results were shown in Fig. 6 and Table 2. Fig. 6a clearly showed a very poor fit between the mesh plot and experimental data points of the Pd/ Cd-DIECCNs for Cd(Ⅱ) since most data points lay up the mesh plot. However, Fig. 6b denoted a very good fitting between the model and experimental data of the Pd/Cd-DIECCNs for Pb(Ⅱ). Furthermore, the higher R2 value for Pb(Ⅱ) than that for Cd(Ⅱ) indicated that the Extended Langmuir isotherm model was much applicable to represent adsorption experimental data of the Pd/ Cd-DIECCNs for Pb(Ⅱ).

|
Download:
|
| Fig. 6. Three-dimensional isotherm surfaces simulated with the extended Langmuir isotherm model (The symbols are the experimental data): (a) for Cd(Ⅱ); (b) for Pb(Ⅱ). | |
|
|
Table 2 Extended Langmuir isotherm models parameters for the adsorption of Pb(Ⅱ) and Cd(Ⅱ) ions from their binary system. |
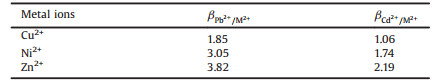
The mixed solutions prepared by solving chloride salts of Pb(Ⅱ), Cd(Ⅱ), Cu(Ⅱ), Ni(Ⅱ) and Zn(Ⅱ) were used to perform an experiment of selective adsorption. The selectivity coefficients and the distribution of Pb(Ⅱ) or Cd(Ⅱ) with respect to Cu(Ⅱ), Ni(Ⅱ) and Zn(Ⅱ) can be obtained from the following equation [32]:
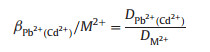
|
where βPb2+(Cd2+) is the selectivity coefficient. M(Ⅱ) represents Cu(Ⅱ), Ni(Ⅱ) or Zn(Ⅱ) ions. D is the distribution ratio, which can be determined as follow:
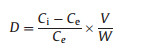
|
where Ci and Ce are the concentrations of metal ions in the initial solution and equilibrium solution (mg/L), respectively; V is the volume of aqueous solution (L) and W is the mass of the dry Pd/Cd-DIECCNs (g). The relative selectivity coefficients of the Pd/Cd-DIECCNs for each individual cationic ion were greater than 1, as shown in Table 3. This means that the Pd/Cd-DIECCNs had good adsorption selectivity for Pb (Ⅱ) or Cd(Ⅱ) in the coexistence system containing other ions. The excellent selectivity is attributed to the specific recognition cavitiesfor the target ions (Pb(Ⅱ) or Cd(Ⅱ)) created in the Pd/Cd-DIECCNs, which are developed by ion imprinting.The results also show that the Pd/CdDIECCNs can be used to enrich Pb(Ⅱ) or Cd (Ⅱ) selectively in the aqueous system containing various metal ions.
|
|
Table 3 Selective adsorption of the Pd/Cd-DIECCNs. |
In summary, Pd/Cd-DIECCNs were prepared by using the electrospinning and ion-imprinting methods from chitosan with Pb(Ⅱ) and Cd(Ⅱ) as templates and glutaraldehyde as a crosslinker. They were used to investigate for adsorption of Pb(Ⅱ) and Cd(Ⅱ) in an aqueous medium. The maximum adsorption capacities of the Pd/Cd-DIECCNs could arrived at 567 mg/g (for Pb(Ⅱ)) and 341 mg/g (for Cd(Ⅱ)) at pH 6.0; the ratio between Pb(Ⅱ) and Cd(Ⅱ) in the mixed solutions was 2:1; the contact time was 8 h; the Pd/CdDIECCNs dose was 30 mg; the temperature was 30 ℃. Adsorption data of the Pd/Cd-DIECCNs for Pb(Ⅱ) was fitted to the Extended Langmuir isotherm significantly better than those for Cd(Ⅱ). The adsorption capacities for different metal ions followed the sequence of QPb(II) > QCd(II) > QCu(II) > QZn(II) > QNi(II), which revealed that the prepared Pd/Cd-DIECCNs had good selectivity for Pb(Ⅱ) and Cd(Ⅱ). In view of these, Pd/Cd-DIECCNs proved to be better candidates among other conventional chitosan materials (flakes or granules) in environmental remediation and they can also be used in water filter or as membrane filters to remove Pb(Ⅱ), Cd(Ⅱ) and even other toxic metal ions.
AcknowledgmentThis work was supported by open project of Key Laboratory of Synthetic and Self-Assembly Chemistry for Organic Functional Molecules (No. K2017-8).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.11.005.
| [1] |
G.F. Nordberg, B.A. Fowler, M. Nordberg, L.T. Friberg, Handbook of the Toxicology of Metals, Academeic Press, London, 2007, pp. 213-249.
|
| [2] |
Y. Al-degs, M.A.M. Khraishen, M.F. Tutunji, Water Res. 35 (2001) 3724-3728. DOI:10.1016/S0043-1354(01)00071-9 |
| [3] |
L.L. Fan, C.N. Luo, M. Sun, X.J. Li, H.M. Qiu, Colloids Surf. B:Biointerfaces 103 (2013) 523-529. DOI:10.1016/j.colsurfb.2012.11.006 |
| [4] |
W.G. Cui, X.H. Li, S.B. Zhou, J. Weng, Appl. Polym. Sci. 103 (2007) 3105-3112. |
| [5] |
W. Ngah, C.S. Endud, R. Mayanar, React. Funct. Polym. 50 (2002) 181-190. DOI:10.1016/S1381-5148(01)00113-4 |
| [6] |
S.P. Wu, X.Z. Dai, J.R. Kan, F.D. Shilong, M.Y. Zhu, Chin. Chem. Lett. 28 (2017) 625-632. DOI:10.1016/j.cclet.2016.11.015 |
| [7] |
Y.S. Zhou, D.Z. Yang, J. Nie, Chin. Chem. Lett. 18 (2007) 118-120. DOI:10.1016/j.cclet.2006.11.035 |
| [8] |
S. Haider, S.Y. Park, J. Membr. Sci. 328 (2009) 90-96. DOI:10.1016/j.memsci.2008.11.046 |
| [9] |
R. Bassi, S.O. Prasher, B.K. Simpson, Sep. Sci. Technol. 35 (2000) 547-560. DOI:10.1081/SS-100100175 |
| [10] |
A.H. Chen, C.Y. Yang, C.Y. Chen, C.Y. Chen, C.W. Chen, J. Hazard. Mater. 163 (2009) 1068-1075. DOI:10.1016/j.jhazmat.2008.07.073 |
| [11] |
N. Sankararamakrishnan, A.K. Sharma, R. Sanghi, J. Hazard. Mater. 148 (2007) 353-359. DOI:10.1016/j.jhazmat.2007.02.043 |
| [12] |
D. Rahangdale, A. Kumar, Carbohydr. Polym. 202 (2018) 334-344. DOI:10.1016/j.carbpol.2018.08.129 |
| [13] |
M. Aliabadi, M. Irani, J. Ismaeili, H. Piri, M.J. Parnian, Chem. Eng. J. 220 (2013) 237-243. DOI:10.1016/j.cej.2013.01.021 |
| [14] |
B.J. Liu, D.F. Wang, Y. Xu, G.Q. Huang, J. Mater. Sci. 46 (2011) 1535-1541. DOI:10.1007/s10853-010-4958-6 |
| [15] |
Y.C. Lu, J. He, G.S. Luo, Chem. Eng. J. 226 (2013) 271-278. DOI:10.1016/j.cej.2013.04.078 |
| [16] |
Y. Li, T.B. Qiu, X.Y. Xu, Eur. Polym. J. 49 (2013) 1487-1494. DOI:10.1016/j.eurpolymj.2013.04.002 |
| [17] |
Y. Li, C. Xu, T.B. Qiu, X.Y. Xu, J. Nanosci. Nanotechnol. 15 (2015) 4245-4254. DOI:10.1166/jnn.2015.10197 |
| [18] |
F.L. Dickert, P. Achatz, K. Halikias, Fresenius J. Anal. Chem. 371 (2001) 11-15. DOI:10.1007/s002160100955 |
| [19] |
F.Z. Xie, H. Xuan, Y. Ge, et al., Chin. J. Anal. Chem. 39 (2011) 77-81. |
| [20] |
F. Xie, G. Liu, F. Wu, G. Guo, G. Li, Chem. Eng. J. 18 (2012) 372-380. |
| [21] |
E. Birlik, A. Ersöz, A. Denizli, R. Say, Anal. Chim. Acta 565 (2006) 145-151. DOI:10.1016/j.aca.2006.02.051 |
| [22] |
S.L. Wei, Y. Liu, M.D. Shao, et al., RSC. Adv. 4 (2014) 29715-29723. DOI:10.1039/C4RA01948B |
| [23] |
M. Aliabadi, M. Irani, J. Ismaeili, S. Najafzadeh, J. Taiwan Inst. Chem. Eng. 45 (2014) 518-526. DOI:10.1016/j.jtice.2013.04.016 |
| [24] |
Y. Li, J. Zhang, Asian Pacific Conference on Energy 2nd, Environment and Sustainable Development 8 (2015) 301-307. |
| [25] |
Y. Liu, Y.R. Kang, D.J. Huang, et al., J. Chem. Technol. Biotechnol. 87 (2012) 1010-1016. DOI:10.1002/jctb.v87.7 |
| [26] |
S. Yeşim, T. Kutsal, Process Biochem. 31 (1996) 573-585. DOI:10.1016/S0032-9592(96)00003-9 |
| [27] |
Q. Li, S. Wu, G. Liu, et al., Sep. Purif. Technol. 34 (2001) 135-142. |
| [28] |
R. Apiratikul, P. Pavasant, Chem. Eng. J. 119 (2009) 135-145. |
| [29] |
V.C. Srivastava, I.D. Mall, I.M. Mishra, J. Hazard. Mater. 134 (2006) 257-267. DOI:10.1016/j.jhazmat.2005.11.052 |
| [30] |
K.M. Al-Qahtani, World Appl. Sci. J. 16 (2012) 465-473. |
| [31] |
A.E. Ofomaja, E.I. Unuabonah, N.A. Oladoja, Bioresour. Technol. 101 (2010) 3844-3852. DOI:10.1016/j.biortech.2009.10.064 |
| [32] |
D.K. Singh, S. Mishra, Anal. Chim. Acta 644 (2009) 42-47. DOI:10.1016/j.aca.2009.04.020 |
 2019, Vol. 30
2019, Vol. 30