Being an important intermediate for the production of terephthalic acid, dimethyl terephthalate and phthalic anhydride [1], xylene, produced by alkylation of benzene with methanol, is receiving increasing attention in the petrochemical industry [2, 3]. ZSM-5 zeolite, as a solid acid catalyst with a 10-membered ring channel structure and mid-strength acidity, has already been widely used and showed high catalytic activity and selectivity for alkylation of toluene and benzene as-reported recently [4]. However, the presence of sole micropores in conventional ZSM- 5 limited the diffusion of bulky substrates, which led to low benzene conversion and selectivity to xylene [5-7].
In order to eliminate the effects of diffusion limitation, the method for conversional zeolite catalysts preparation was modified by introducing mesoporous into the zeolite catalyst. As expected, the hierarchical porous ZSM-5 catalyst achieved excellent catalytic properties in. benzene alkylation reactions [5]. However, the main side-product olefins unavoidably, were produced even over the hierarchical porous ZSM-5, which resulted in the formation of ethylbenzene and coke and thus degraded catalysts [6]. It was said that the introduction of metals with high dehydrogenation capacity such as Pt and Pd in some degree mitigates the coke and ethylbenzene formation. The noble metal incorporated zeolite catalyst, however, received little attention in view of industrial applications, possibly due to the high cost of noble metal itself. Fortunately, Lu et al. and Hu et al. introduced non-noble basic metals Mg and Zn into hierarchical porous ZSM-5 catalysts, which have been verified to be able to promote conversion of benzene to xylene. Lyu et al. synthesized nitrided hierarchical porous ZSM-5 by nitridation of hierarchical porous ZSM-5 with flowing ammonia at elevated temperature. The result showed that nitridation adjusted the Brönsted acidity effectively and the high suppression of ethylbenzene was observed [8]. This is because the presence of strong Brönsted acidity of the ZSM-5 catalyst was responsible for the methanol to olefins reaction. The introduction of basic Mg and Zn dramatically decreased the number of Brönsted acidity of ZSM-5, which thus suppressed the formation of the side-product olefins and promoted catalytic performance for benzene alkylation instead [9-13]. More recently, our group observed that changing the Si/Al ratio of hierarchical porous ZSM-5 in the catalyst synthesis process was also a promising method, which could affect the Brönsted acid dispersion and intensity. As a result, the catalyst with high Si/Al ratio (1800) effectively inhibited the formation of ethylbenzene and enhanced the selectivity to xylene without the addition of a noble metal [14]. However, in order to obtain better catalytic performance, here, we synthesized hierarchical porous ZSM-5 catalyst with Si/Al ratio of 1800 by solvent evaporation assisted dry gel conversion method and used it for continuous catalytic conversion of benzene alkylation with methanol to xylene. The reaction conditions including temperature, pressure, benzene/methanol molar ratio and weight hour space velocity (WHSV) were primarily investigated and optimized, since they played an important role in the alkylation of aromatics [9, 15-19]. In addition, the textural properties of synthesized materials were characterized by various techniques (including XRD, BET, IR, TPD, SEM and TEM).
The high-silica hierarchical porous ZSM-5 catalyst and microporous ZSM-5 (Si/Al = 1800) was prepared via solvent evaporation assisted dry gel conversion route [20]. Typically, the homogeneous solution which is consist of five kinds of raw materials including tetraethylorthosilicate (TEOS), aluminum isopropoxide (AIP), tetra-n-propylammonium hydroxide (TPAOH), hexadecyltrimethoxysilane (HTS) and ethanol (EtOH) with a molar ratio of 1SiO2:0.00028Al2O3:0.2TPAOH:xHTS:15EtOH was prepared (x = 0, 0.05). The resultant gel mixture was aged at room temperature for 24 h and placed into a PTFE cup before being transferred into a Teflon-lined autoclave. A small amount of water was added outside the PTFE cup to create steam for the hydrothermal synthesis conditions. Then, the stainless steel autoclave was heated at 180 ℃ for 72 h. After crystallization finished, the product was subjected to be filtrated without ionexchange steps and calcined at 550 ℃ for 7 h with the heating rate of 10 ℃/min to obtain H-form hierarchical porous ZSM-5. The conventional ZSM-5 (Si/Al = 19) was bought from Nankai University Catalyst Co., Ltd.
The catalysts were characterized using a transmission electron microscopy (TEM) (Tecnai G2 F30 S-Twin) and scanning electron microscopy (SEM) (Hitachi S-4700(Ⅱ)). X-ray diffraction (XRD) (SCINTAG X" TRA) was measured using Cu K-radiation (1542A) at 30 mA and 40 kV with a scanning angle (2θ) from 5° to 80°. The nitrogen absorption isotherms were conducted at 77 K (Quantachrome, NOVA 1000e). The NH3-TPD was carried out on Tianjin XQ TP-5076 chemisorption instrument. And Py-IR was operated on Nicolet iS-500.
All evaluation experiments were carried out in a continuousflow fixed-bed reactor with a stainless steel tube (8 mm i.d.) under atmospheric pressure. In each test, 0.5 g of catalyst diluted with 5.0 g inert quartz sand was loaded in the middle of the tube reactor. The catalyst was heat-treated in situ from ambient temperature to 380–520 ℃ at 10 ℃/min in N2 flow with a space velocity of 2400 h-1. Then the mixture of benzene and methanol mixture was fed into the reactor with a co-feed N2 flow of 40 mL/min. The effluent from the reactor was analyzed online by the gas chromatography (Fuli GC9790) with a DB-1 capillary column (30 m × 0.25 mm × 1.00 mm) and a flame ionization detector. In order to ensure all of the products were in gas phase, the temperature of the effluent line was maintained at 200 ℃ by a heating belt.
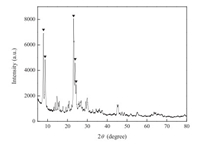
Fig. 1 showed the XRD pattern of the synthesized hierarchical porous ZSM-5 catalyst sample. The patterns observed at 2θ of 7.9°, 8.8°, 23.1°, 23.9°, and 24.3° matched nicely with the standard card and those of the ZSM-5 (MFI) structure reported in the literature [21, 22], suggesting that the ZSM-5 zeolite was obtained successfully.
|
Download:
|
| Fig. 1. XRD patterns of synthesized hierarchical porous ZSM-5 catalysts. | |
The SEM and TEM images of synthesized high-silica hierarchical porous ZSM-5 were given in Fig. 2. It could be seen that the shape of the sample crystals was roughly ellipsoidal with a size of about 200–300 nm (Figs. 2a and b). The TEM images (Fig. 2c) clearly suggested that one single secondary particle aggregate was made up of abundant primary nanozeolites with an average diameter of about 10–50 nm. The high magnification TEM images (Fig. 2d) revealed that the nanoparticles were crystalline in nature. These results were consistent with the literature reported by Zhu et al. and Hu et al. [14, 20].

|
Download:
|
| Fig. 2. SEM image (a, b) and TEM image (c, d) of synthesized hierarchical porous ZSM-5. | |
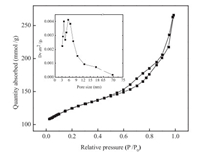
As shown in Fig. 3, the N2 physisorption isotherm of the synthesized hierarchical porous ZSM-5 was a typical type-Ⅳ isotherm, which has a hysteresis loop that was associated with capillary condensation taking place in the mesopores. The total surface areas were determined by the Brunauer-Emmett-Teller (BET) method and the surface area was 454 m2/g. The Barrett– Joyner–Halenda (BJH) model was used to calculate the pore size distribution, from which the size of the mesopores was centered at 6 nm, while the pore size of conventional ZSM-5 was around 1.2 nm, which was presented in Fig. S2 in Supporting information. In addition, the peak at around 4 nm was attributed to the TSE phenomenon [23].
|
Download:
|
| Fig. 3. Nitrogen adsorption-desorption isotherms of the synthesized hierarchical porous ZSM-5 (inset: pore size distribution deduced from the adsorption branch of the isotherm). | |
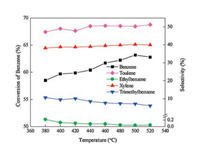
Fig. 4 exhibited the effect of temperature on the conversion of benzene and the selectivity to xylene over high-silica hierarchical porous ZSM-5. The benzene conversion was increased from 57.4% to 63.2% as the temperature increased from 380 ℃ to 500 ℃, indicating that appropriate increasing in reaction temperature was conducive to enhance the catalytic activity of catalyst, which had been already illustrated by Kaeding [24] and Odedairo et al. [25]. Apart from great influence on benzene conversion, the temperature highly affected the selectivity of products as well. As the temperature increased from 380 ℃ to 500 ℃, the selectivity to toluene and xylene increased from 47.9% to 50.3% and 37.8% to 40.1%, respectively. The selectivity to trimethylbenzene, however, decreased from 10.% to 7.15% and the selectivity to ethylbenzene was maintained below 0.1%. As the reaction temperature increased beyond 500 ℃, the benzene conversion decreased slightly. This could be attributed to a fact that the alkylation of benzene with methanol was exothermic. Therefore the high temperature was unfavorable for these main reactions [10]. Moreover, the MTO reactions would be promoted greatly at higher temperature [19].
|
Download:
|
| Fig. 4. Effects of temperature on the conversion of benzene and selectivity to products over high-silica hierarchical porous ZSM-5 (0.1 MPa, WHSV = 2 h-1, time on stream = 10 h, benzene/methanol molar ratio = 1). | |
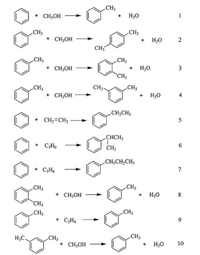
Fig. 5 depicted the main relationship between reaction temperature and Gibbs energy involved in alkylation of benzene (Scheme 1) [26]. As shown in Fig. 5, with the increase of reaction temperature (350–500 ℃), the Gibbs energy of the alkylation of benzene with methanol to produce toluene and xylene decreased gradually. Noticeably, the Gibbs energy of these two reactions was all less than zero [27], suggesting the fact that these two reaction pathways proceeded spontaneously and could be promoted by increasing the reaction temperature. As for the reaction of trimethylbenzene and ethylbenzene formation, the Gibbs energy all increased with the increase of temperature, revealing that the high temperature was unfavorable for both two side reactions, which is well explained by the catalytic result obtained in Fig. 4. Although increasing temperature enhanced the methanol selfreactions (Fig. 6, Scheme 2), however, the high-silica hierarchical porous ZSM-5 itself is a kind of catalyst with high ability to suppress the formation of ethylbenzene, so the selectivity to ethylbenzene was maintained below 0.1% [14].

|
Download:
|
| Fig. 5. Relation of temperature and Gibbs energies of the reactions [26]. | |
|
Download:
|
| Scheme 1. Benzene alkylation with methanol [26]. | |

|
Download:
|
| Fig. 6. Relation of temperature and Gibbs energies of the reactions [30]. | |
|
Download:
|
| Scheme 2. Methanol self-reactions [30]. | |
Liu [28] had investigated the same reaction over the conventional ZSM-5 with Si/Al molar ratio of 60 varying the temperature from 350 ℃ to 480 ℃. As a result, the temperature of 460 ℃ was more active for alkylation of benzene with almost 50.35% benzene conversion and 36.03% selectivity to xylene achieved. The selectivity to toluene and xylene, however, decreased while the benzene conversion still keep increasing, which is consistent with the results reported by the others [10, 29]. Based on the work done in our group, the temperature of 500 ℃ was considered as the optimum reaction temperature over high-silica hierarchical porous ZSM-5 for benzene alkylation with methanol (Fig. 6, Scheme 1).
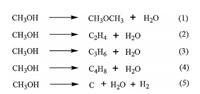
Fig. 7 illustrated the effect of reaction pressure on the conversion of benzene and the selectivity to products over highsilica hierarchical porous ZSM-5. It could be seen that with the increase of reaction pressure from 0.1 MPa to 0.3 MPa, the conversion of benzene and the main products selectivity (toluene and xylene) changed slightly, indicating that the pressure may not be a positive factor influencing the catalytic activity of catalyst. The similar phenomenon had been observed by Kaeding et al. [24]. However, it should be noted that the selectivity to ethylbenzene was increased obviously after increasing the pressure. It was because that increasing the pressure would promote the alkylation of benzene with ethylene which was a molecular decreasing reaction [31]. Therefore, according to the result obtained in Fig. 7, we concluded that the pressure of 0.1 MPa was the optimal pressure for benzene alkylation with methanol (Scheme 2) [30].
|
Download:
|
| Fig. 7. Effect of pressure on conversion and selectivity to products over high-silica hierarchical porous ZSM-5 (reaction temperature 500 ℃, WHSV = 2 h-1, benzene/ methanol molar ratio = 1, time on stream = 10 h). | |
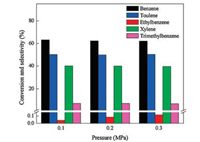
The effect of benzene/methanol molar ratio on the benzene conversion and the selectivity to xylene was also investigated. As shown in Fig. 8, with the decrease of benzene/methanol molar ratio from 2:1 to 1:1.5, the benzene conversion increased dramatically from 39.5% to 82.1%, while the selectivity to xylene and trimethylbenzene increased gradually from 26.9% to 50.2% and 2.6% to 12.3%, respectively.
|
Download:
|
| Fig. 8. Effect of benzene/methanol ratio on the conversion of benzene and selectivity to products over high-silica hierarchical porous ZSM-5 (500 ℃, 0.1 MPa, WHSV = 2 h-1, time on stream = 10 h). | |
Meanwhile, the selectivity to toluene gradually went down from 69.0% to 34%. In benzene alkylation with methanol, the formation of toluene, xylene and trimethylbenzene represented the primary, secondary and tertiary alkylation reaction, respectively [15]. At the highest benzene/methanol molar ratio of 2:1, methanol was the limiting reagent which limited the conversion of benzene and the further conversion of toluene which therefore led to the lower benzene conversion and the higher selectivity to toluene. With the decrease of benzene/methanol molar ratio, the amount of methanol introduced increased and benzene conversion and selectivity to xylene were increased as well. Noticeably, the selectivity to trimethylbenzene was increased at the same time, indicating that the tertiary alkylation step was also promoted. As decreased the benzene/methanol molar ratio to 1:2, the increase of selectivity to xylene was slight while the increase of selectivity to trimethylbenzene was obvious, suggesting that the much excess amount of reactant methanol mainly promoted the depth alkylation. Considering the figure of xylene was higher than that of trimethylbenzene, the benzene/methanol molar ratio of 1:1.5 was considered as the optimum one [9, 18].
It was worth noting that the benzene/methanol molar ratio of 1:1 was used in many reported literatures and amount of methanol introduced into the reactor was strictly controlled in order to reduce the formation of side-products. Because the side reaction of methanol to olefins would be intensified if excess methanol was introduced and this in turn promoted the formation of by-products ethylbenzene [9-12, 29, 30]. Fortunately, in this work, the selectivity to ethylbenzene was always less than 0.1% during changing the molar ratio (Fig. 8), over the high-silica hierarchical porous ZSM-5 catalyst, indicating that the side reactions of methanol to olefins were successfully suppressed. There are two reasons for this phenomenon. The first reason was that the high-silica hierarchical porous ZSM-5 catalyst with appropriate Brönsted acidity highly suppressed the side reaction of methanol [14]. The other was the presence of mesopores in catalyst, which promoted the diffusion of bulky reactants and products and this made it easy for alkylation of aromatics with methanol [6]. Based on the two aspects discussed above, the high-silica hierarchical porous ZSM-5 catalyst with appropriate Brönsted acidity and special mesoporous structure is more active for alkylation of benzene.
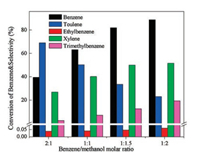
As shown in Fig. 9, with the increase of the WHSV from 1 h-1 to 4 h-1, the conversion of benzene increased slightly from 81% to 82%, and the same trend for selectivity to toluene and xylene was observed, the figure only rising from 33.5% to 34.3% and 49.4% to 50.5%, respectively. At low WHSV, the contact time of reactants and catalyst was long which enhanced the side reactions such as disproportionation, isomerization and depth alkylation [29]. Moreover, as for the MTO reaction, the low WHSV could increase the possibility for the formation of high olefins, which resulted in the low conversion of benzene and selectivity to xylene [32]. Therefore, the increase of WHSV in optimum value, to some extent, could help to suppress the side reactions and improve the benzene conversion and xylene selectivity. However, further increasing the WHSV to 8 h-1, the contact time of reactants and catalyst was too short to react, which limited the alkylation of benzene and toluene, and led to the low benzene conversion and selectivity to xylene. In order to obtain the high yield of xylene, The WHSV of 4 h-1 was considered as the optimum. After the optimization of above conditions, the yield of xylene was increased from 21.1% to 41.4% which significantly improve the economic benefit of the alkylation process. According to the literature, the optimal WHSV of 2 h-1 was mainly reported over the conventional ZSM-5 [28, 30] while 4 h-1 in this work, this was because of the fact that the introduction of mesoporous promoted the diffusion of bulky reactants in the reaction of benzene alkylation with methanol [6].
|
Download:
|
| Fig. 9. Effect of WHSV on the conversion of benzene and selectivity to products over high-silica hierarchical porous ZSM-5 (500 ℃, 0.1 MPa, benzene/methanol molar ratio = 1:1.5, time on stream = 10 h). | |
For better comparison, the commercial conventional ZSM-5 and high-silica microporous ZSM-5(Si/Al = 1800) were also tested. As shown in Figs. S5 and S6 (Supporting information), the benzene conversion and selectivity to xylene over the high-silica hierarchical porous ZSM-5 were much higher than the other two catalysts. These were because of the optimized acidity and the diffusion effect of the hierarchical porous structure [8, 11, 12, 14, 33], which could be investigated in the results of nitrogen adsorption-desorption, NH3-TPD and Py-IR (Figs. S2–S4 in Supporting information).
The high-silica hierarchical porous ZSM-5 was synthesized by sol-gel method for benzene alkylation with methanol to xylene. The effects of temperature, pressure, benzene/methanol molar ratio and weight hour space velocity (WHSV) on the catalytic performance of catalyst were investigated. Consequently, the highsilica hierarchical porous ZSM-5 showed great performance with the yield of xylene was up to 41.1% under the optimum reaction conditions (500 ℃, 0.1 MPa, Mbenzene/Mmethanol = 1:1.5 and WHSV = 4 h-1), while the yield to by-products of ethylbenzene was well suppressed (below 0.1%). This could be ascribed to the fact that the high-silica hierarchical porous ZSM-5 catalyst with appropriate Brönsted acidity for benzene alkylation with methanol suppressed the side reactions of methanol, and the hierarchical porous structure promoted the diffusion of alkyl aromatics.
AcknowledgmentsWe acknowledge financial support from the National Natural Science Foundation of China (Nos. NSFC-21476207 and NSFC- 21506189) and National Basic Research Program of China (973 Program, No. 2011CB710800).
Appendix A. Supplementary dataSupplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.cclet.2018.09.016
| [1] |
W. Alabi, L. Atanda, R. Jermy, et al., Chem.-Eng. J. 195- 196 (2012) 276-288. |
| [2] |
A.A. Rownaghi, J. Hedlund, Ind. Eng. Chem. Res. 50 (2011) 11872-11878. DOI:10.1021/ie201549j |
| [3] |
X.M. Wang, J. Xu, G.D. Qi, et al., J. Phys. Chem. C 117 (2013) 4018-4023. DOI:10.1021/jp310872a |
| [4] |
Z.R. Zhu, Q.L. Chen, Z.K. Xie, et al., Micropor. Mesopor. Mater. 88 (2006) 16-21. DOI:10.1016/j.micromeso.2005.08.021 |
| [5] |
C.H. Christensen, K. Johannsen, I. Schmidt, et al., J. Am. Chem. Soc. 125 (2003) 13370-13371. DOI:10.1021/ja037063c |
| [6] |
H.L. Hu, Q.F. Zhang, J. Cen, et al., Catal. Lett. 145 (2015) 715-722. DOI:10.1007/s10562-014-1458-3 |
| [7] |
W. Deng, X. He, C. Zhang, et al., Chin. J. Chem. Eng. 22 (2014) 921-929. DOI:10.1016/j.cjche.2014.06.009 |
| [8] |
J.H. Lyu, H.L. Hu, J.Y. Rui, et al., Chin. Chem. Lett. 28 (2017) 482-486. DOI:10.1016/j.cclet.2016.10.025 |
| [9] |
M.O. Adebajo, M.A. Long, Catal. Commun 4 (2003) 71-76. DOI:10.1016/S1566-7367(02)00259-5 |
| [10] |
L. Lu, H.Z. Zhang, X.D. Zhu, Acta Pet. Sin. 2 (2012) 111-115. |
| [11] |
H.L. Hu, J.H. Lyu, Q.T. Wang, et al., RSC Adv. 5 (2015) 32679-32684. DOI:10.1039/C5RA03048J |
| [12] |
H.L. Hu, J.H. Lyu, J. Cen, et al., RSC Adv. 5 (2015) 63044-63049. DOI:10.1039/C5RA12589H |
| [13] |
S.F. Zhao, X.T. Yao, B.H. Ya, et al., Chin. Chem. Lett. 28 (2017) 1318-1332. DOI:10.1016/j.cclet.2017.03.023 |
| [14] |
H.L. Hu, J.H. Lyu, J.Y. Rui, et al., Catal. Sci. Technol. 6 (2016) 2647-2652. DOI:10.1039/C5CY01976A |
| [15] |
M.O. Adebajo, R.F. Howe, M.A. Long, Catal. Today 63 (2000) 471-478. DOI:10.1016/S0920-5861(00)00493-4 |
| [16] |
S. Al-Khattaf, C. D'Agostino, M.N. Akhtar, et al., Catal. Sci. Technol. 4 (2014) 1017-1027. DOI:10.1039/c3cy00925d |
| [17] |
J.P. Breen, R. Burch, M. Kulkarni, et al., Appl. Catal. A Gen. 316 (2007) 53-60. DOI:10.1016/j.apcata.2006.09.017 |
| [18] |
M.Y. Huan, H.M. Sun, B. Zhang, et al., Chem. React. Eng. Technol. 5 (2008) 395-399. |
| [19] |
J.H. Gao, L.D. Zhang, J.X. Hu, et al., Chem. Ind. Eng. Process. 11 (2008) 1800-1804. |
| [20] |
K.K. Zhu, J.M. Sun, J. Liu, et al., ACS Catal. 1 (2011) 682-690. DOI:10.1021/cs200085e |
| [21] |
A. Gervasini, Appl. Catal. A-Gen. 180 (1999) 71-82. DOI:10.1016/S0926-860X(98)00333-0 |
| [22] |
H. van Koningsveld, J.C. Jansen, H.V. Bekkum, Zeolites 10 (1990) 235-242. DOI:10.1016/0144-2449(94)90134-1 |
| [23] |
J.C. Groen, L.A.A. Peffer, J. Perez-Ramirez, Micropor. Mesopor. Mater. 60 (2003) 1-17. DOI:10.1016/S1387-1811(03)00339-1 |
| [24] |
W.W. Kaeding, J. Catal. 114 (1988) 271-276. DOI:10.1016/0021-9517(88)90030-9 |
| [25] |
T. Odedairo, S. Al-Khattaf, Catal. Today 204 (2013) 73-84. DOI:10.1016/j.cattod.2012.05.052 |
| [26] |
Y.R. Xu, X.L. Xu, X.D. Zhu, Chem. React. Eng. Technol. 5 (2015) 475-480. |
| [27] |
S. Hajimirzaee, M. Ainte, B. Soltani, et al., Chem. Eng. Res. Des. 93 (2015) 541-553. DOI:10.1016/j.cherd.2014.05.011 |
| [28] |
J. Liu, Chem. Prod. Technol. 18 (2011) 19-21. |
| [29] |
S.L. Zhang, L.L. Zhang, W.G. Wang, et al., Acta Phys. Chim. Sin. 3 (2014) 535-543. |
| [30] |
L. Wang, J.L. Zhu, H.B. Li, et al., Chem. Eng. 36 (2008) 48-51. |
| [31] |
E. Aghaei, M. Haghighi, Powder Technol. 269 (2015) 358-370. DOI:10.1016/j.powtec.2014.09.036 |
| [32] |
J. Liu, Polyester Ind. 28 (2015) 18-20. |
| [33] |
J.H. Lyu, H.L. Hu, C. Tait, et al., Chin. J. Chem. Eng. 25 (2017) 1187-1194. DOI:10.1016/j.cjche.2016.12.005 |
 2019, Vol. 30
2019, Vol. 30 

