b University of Chinese Academy of Sciences, Beijing 100049, China;
c Shanghai Research Institute of Fragrance and Flavor Industry, Shanghai 200232, China;
d School of Perfume and Aroma Technology, Shanghai Institute of Technology, Shanghai 200233, China
Perfumes are widely used in food [1, 2], cosmetics [3, 4], detergents [5, 6], and so on. In particular, they have made great contributions to creating a clean and fresh healthy air environment [7, 8]. Rather than product air freshener, add the perfume to everyday objects for more convenient and comfortable life, such as wallpaper. However, A large number of fragrances are prone to oxidation [9, 10], and hydrolysis [11, 12], especially volatilization [13, 14]. The rapid release severely influences the storage, application and quality of these perfumes. Therefore, it is meaningful to find ways to overcome this obstacle.
With the development of nanotechnology, there have been plenty of physicochemical strategies to slow the release rate [15-19]. The fragrances could be encapsulated into nanomaterials and released slowly due to the interactions between fragrances and nanomaterials. The interactions mainly include electrostatic interaction, hydrophilic and hydrophobic interaction, covalent interaction, coordination interaction, interfacial interaction, and so on. But only slowing release rate of aromas are not enough. Fragrances do not need to be released all the time. In other words, they need to be released controllably as needed.
For the fragrances in the wallpaper, it need not be released in the dark. Because it was stored or we are sleep at this point. So the light-induced nanomaterials need to be designed to encapsulate and controllably release perfumes. Zhao and his colleagues developed many kinds of photo-responsive block copolymer micelles [20-23]. These micelles could be disrupted irreversibly in the light and release their cargos. Many photo-responsive gels were also created [24-27]. These gels could be induced to swell, shrink and disrupt, and release the molecules they encapsulated. However, these photo-responsive materials were either degraded irreversibly, or swelled and shrunken not rapid enough. Therefore, it is necessary and urgent to develop a reversible and sensitive light-induced nanomaterial for the fragrance.
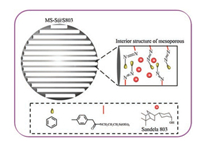
To satisfy these conditions, a photo-activated mesoporous silica loaded with sandela 803 was designed, prepared and named as S803@MS-S. As shown in Fig. 1, due to the large specific surface area and hydrophobic azobenzene group, hydrophobic sandela 803 was adsorbed into the mesoporous of S803@MS-S via interfacial interaction and hydrophobic interaction [28-30]. In the dark, the azobenzene derivatives seemed like gatekeepers to the sandela 803 and prevented the odorant molecules from escaping [31]. In the light, the dynamic motion of azobenzene derivatives could be caused by photoinduced cis-trans isomerization of N=N bonds [32]. The azobenzene derivatives was thus regarded as the impellers to impel the guest molecules from the mesoporous [31]. That is to say, S803@MS-S could controllably release the sandela 803 in the light and protected them from the loss in the dark.
|
Download:
|
| Fig. 1. Schematic diagram of the S803@MS-S and the interior structure of the mesoporous. | |
The photo-responsive silane coupling agent TESPIC-AZO was synthesized via an addition reaction (Fig. S1A in Supporting information). The molecular weight of the obtained product was detected by FTICR-MS. As shown in Fig. S1B (Supporting information), there was a peak of 445.13, which was peak of ICPES-AZO. So ICPES-AZO was synthesized successfully.
In the following, mesoporous silica nanospheres were prepared. Firstly, with the surfactant of CTAB as liquid crystal templating, TEOS as silica precursor, and alkali as catalyst, pure mesoporous silica nanospheres were prepared via the sol-gel method. ICPES-AZO was then attached into pure mesoporous silica nanosphere to prepared photo-responsive mesoporous silica nanospheres (MS-S).
The morphology of MS-S was observed via SEM. As shown in Fig. 2A, the morphology and size of MS-S were homogeneous. All the MS-S was spherical and the diameter of MS-S was about 100nm. And the surface of the MS-S was a little bit rough. The N2 adsorption-desorption isotherms of the MS-S was detected. As shown in Fig. 2B, it exhibited a type Ⅳ isothermal line as defined by IUPAC, which indicated that the MS-S possessed mesopores. As shown in Fig. 2C, the pore diameter of MS-S measured via desorption pore volume distribution was 2.642nm. The total pore volume was 1.03 cm3/g, and the BET specific surface area was 1320m2/g, which indicated that MS-S had a high porosity.

|
Download:
|
| Fig. 2. (A) The SEM imageof MS-S. (B) The N2 adsorption-desorption isothermat 77K for MS-S. (C) The porediametersof MS-S. (D) The TGA results.a: sandela 803; b: MS-S; c: S803@M. | |
The MS-S was then used to encapsulate sandela 803. The morphology of S803@MS-S was observed through SEM. As shown in Fig. S2 (Supporting information), the morphology and size remains unchanged after addition of sandela 803. The morphology and size of S803@MS-S were also homogeneous and the diameter of MS-S was about 100nm.
The thermal property and encapsulation efficiency were measured by TGA. As shown in Fig. 2D, sandela 803 was decomposed completely between 120 ℃ and 220 ℃, while MS-S was decomposed between 200 ℃ and 300 ℃. S803@MS-S had two thermal decomposition periods, the first decomposition corresponded to sandela 803, and the second one corresponded to MS-S. The encapsulation efficiency of sandela 803 in S803@MS-S was calculated through TGA results and it was 37.42%.
If S803@MS-S is applied to daily life, it must be biocompatible. And the biocompatibility of S803@MS-S in cellular level was evaluated via MTT assay. The human skin fibroblast (HSF) cells were chosen to analyze it. HSF cells were treated with S803@MS-S S NPs for 24h and 48h. The cell viabilities were then measured. As shown in Fig. S3 (Supporting information), there were no obvious cell death when the concentration of S803@MS-S was up to 0.8 mg/mL, which indicated that the S803@MS-S NPs had an excellent biocompatible property. Therefore, S803@MS-S NPs could be used in daily life.
Wallpaper was then treated with S803@MS-S and named S803@MS-S-W. The morphology of the wallpaper was then observed via SEM. As shown in Fig. 3A, the wallpaper was composed of plant fibers. The surface of untreated wallpaper fibers was smooth. As shown in Fig. 3B, the surface of S803@MS-S became rough. There were a number of silica nanospheres in the wallpaper according to the magnified image of Fig. 3B, which indicated that the 803@ MS-S was adhered on the surface of wallpaper successfully.

|
Download:
|
| Fig. 3. The SEM images of (A) untreated wallpaper and (B) wallpaper treated with S803@MS. | |
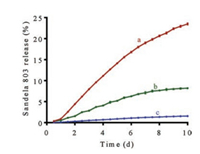
The photo-responsive release property of sandela 803 in S803@MS-S was detected via GC-FID. As shown in Fig. 4, free sandela 803 was rapidly released from the wallpaper. And the release amount of sandela 803 reached 23.49% after 10 days. When the wallpaper treated with S803@MS-S, the release rate of sandela 803 was significantly reduced both in the dark and in the light, which indicated that S803@MS-S had an excellent slow-release effect. When the S803@MS-S-W was in the light, 8.19% of sandela 803 was released from the wallpaper after 10 days. By contrast, only 1.57% of sandela 803 was released in the dark, which indicated that S803@MS-S-W had a distinguished photo-responsive release property. Above all, S803@MS-S-W had excellent sustained and controlled release performances.
|
Download:
|
| Fig. 4. The cumulative release profiles of sandela 803 in different samples. a: wallpaper treated with free sandela 803 in the dark; b: S803@MS-S-W in the light for 12 h and dark for 12 h per day; c: S803@MS-S-W in the dark. The mean ± SD is shown (n = 3). | |
In summary, photo-responsive mesoporous silica nanospheres loaded with sandela 803 was successfully developed and named as S803@MS-S. It possessed ordered and uniform mesoporous. The pore volume and specific surface area were up to 1.03cm3/g and 1320m2/g, respectively. Therefore, the silica nanosphere could adsorb a mass of sandela 803, and the encapsulation efficiency reached 37.42%. In addition, the MTT assay demonstrated that S803@MS-S was biocompatible. So S803@MS-S could applied to daily life. S803@MS-S was then attempted to be added into wallpaper and exhibited excellent sustained and controlled release performances.
AcknowledgmentsThis work was financially supported by the National High Technology Research and Development Program (No. 2016YFA0200303), the Beijing Natural Science Foundation (No. L172046), the National Natural Science Foundation of China (Nos. 31522023, 51373177 and 51573188), the Beijing Municipal Science & Technology Commission (No. Z161100002616015), the "Strategic Priority Research Program Research Program" of the Chinese Academy of Sciences (No. XDA09030301-3).
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi: https://doi.org/10.1016/j.cclet.2018.09.015.
| [1] |
E. Guichard, Food. Rev. Int. 18 (2002) 49-70. DOI:10.1081/FRI-120003417 |
| [2] |
H.H. Jelen, M. Majcher, M. Dziadas, Anal. Chim. Acta 738 (2012) 13-26. DOI:10.1016/j.aca.2012.06.006 |
| [3] |
L. Sanchez-Prado, J.P. Lamas, G. Alvarez-Rivera, et al., J. Chromatogr. A 1218 (2011) 5055-5062. DOI:10.1016/j.chroma.2011.06.013 |
| [4] |
N.Y.O. Muyima, G. Zulu, T. Bhengu, D. Popplewell, Flavour Fragrance J. 17 (2002) 258-266. |
| [5] |
L. Loretz, A.M. Api, L. Barraj, et al., Food Chem. Toxicol. 44 (2006) 2008-2018. DOI:10.1016/j.fct.2006.06.029 |
| [6] |
Y. Chen, F. Begnaud, A. Chaintreau, J. Pawliszyn, Flavour Fragrance J. 21 (2006) 822-832. |
| [7] |
M. Hitosugi, C. Tsukada, S. Yamauchi, et al., Leg. Med. (Tokyo) 17 (2015) 360-363. DOI:10.1016/j.legalmed.2015.04.004 |
| [8] |
X. Zhang, J.A. Teixeira da Silva, M. Niu, et al., Sci. Rep. 7 (2017) 42165. DOI:10.1038/srep42165 |
| [9] |
M. Skold, A. Borje, M. Matura, A.T. Karlberg, Contact Dermatitis 46 (2002) 267-272. DOI:10.1034/j.1600-0536.2002.460504.x |
| [10] |
X. Fernandez, E. Dunach, R. Fellous, et al., Flavour Fragrance J. 17 (2002) 432-439. DOI:10.1002/ffj.v17:6 |
| [11] |
A.A. Al-Gencly, G.B. Lockwood, Flavour Fragrance J. 18 (2003) 148-152. |
| [12] |
E. Pogorzelski, A. Wilkowska, Flavour Fragrance J. 22 (2007) 251-254. |
| [13] |
Z.B. Xiao, L. He, G.Y. Zhu, Flavour Fragrance J. 29 (2014) 350-355. DOI:10.1002/ffj.v29.6 |
| [14] |
A.M. Borda, D.G. Clark, D.J. Huber, B.A. Welt, T.A. Nell, Postharv. Biol. Technol. 59 (2011) 245-252. DOI:10.1016/j.postharvbio.2010.09.008 |
| [15] |
J. Hu, L.Q. Liu, Y.Y. Xie, L.M. Wu, Polym. Chem.-U. K. 4 (2013) 3293-3299. DOI:10.1039/c3py00186e |
| [16] |
Y.L. Zhang, J.M. Pelet, D.A. Heller, Y.Z. Dong, et al., Adv. Mater. 25 (2013) 4641-4645. DOI:10.1002/adma.201301917 |
| [17] |
M. Zandi, M. Mohebbei, Flavour Fragrance J. 29 (2014) 364-370. DOI:10.1002/ffj.v29.6 |
| [18] |
P. Wang, Y.H. Zhu, X.L. Yang, A.P. Chen, Flavour Fragrance J. 23 (2008) 29-34. |
| [19] |
B. Indradas, C. Hansen, M. Palmer, G.B. Womack, Flavour Fragrance J. 29 (2014) 313-323. DOI:10.1002/ffj.v29.5 |
| [20] |
J.F. Gohy, Y. Zhao, Chem. Soc. Rev. 42 (2013) 7117-7129. DOI:10.1039/c3cs35469e |
| [21] |
J.Q. Jiang, X. Tong, Y. Zhao, J. Am. Chem. Soc. 127 (2005) 8290-8291. DOI:10.1021/ja0521019 |
| [22] |
B. Yan, J.C. Boyer, N.R. Branda, Y. Zhao, J. Am. Chem. Soc. 133 (2011) 19714-19717. DOI:10.1021/ja209793b |
| [23] |
S. Kumar, J.F. Allard, D. Morris, et al., J. Mater. Chem. 22 (2012) 7252-7257. DOI:10.1039/c2jm16380b |
| [24] |
M. Czugala, C. O'Connell, C. Blin, et al., Sens. Actuator B-Chem. 194 (2014) 105-113. DOI:10.1016/j.snb.2013.12.072 |
| [25] |
D. Blanc, S. Pelissier, P.Y. Jurine, J. Sol-Gel Sci. Technol. 27 (2003) 215-220. DOI:10.1023/A:1023710903667 |
| [26] |
F. Xie, L. Qin, M.H. Liu, Chem. Commun. 52 (2016) 930-933. DOI:10.1039/C5CC08076B |
| [27] |
D. Costa, A.J.M. Valente, M.G. Miguel, J. Queiroz, Langmuir 27 (2011) 13780-13789. DOI:10.1021/la2026285 |
| [28] |
I.I. Slowing, B.G. Trewyn, S. Giri, V.S.Y. Lin, Adv. Funct. Mater. 17 (2007) 1225-1236. DOI:10.1002/adfm.v17:8 |
| [29] |
J.L. Vivero-Escoto, I.I. Slowing, B.G. Trewyn, V.S.Y. Lin, Small 6 (2010) 1952-1967. DOI:10.1002/smll.200901789 |
| [30] |
T. Koishi, S. Yoo, K. Yasuoka, et al., Phys. Rev. Lett. 93 (2004) 185701. DOI:10.1103/PhysRevLett.93.185701 |
| [31] |
S. Angelos, E. Choi, F. Vogtle, L. De Cola, J.I. Zink, J. Phys. Chem. C 111 (2007) 6589-6592. DOI:10.1021/jp070721l |
| [32] |
N.G. Liu, D.R. Dunphy, M.A. Rodriguez, S. Singer, J. Brinker, Chem. Commun. (2003) 1144-1145. |
 2019, Vol. 30
2019, Vol. 30 

