b University of Chinese Academy of Sciences, Beijing 100049, China;
c Shanghai Research Institute of Fragrance and Flavor Industry, Shanghai 200232, China;
d School of Perfume and Aroma Technology, Shanghai Institute of Technology, Shanghai 200233, China
Fragrance has been widely used in food, household cleaners, tobacco, room sprays, cosmetics, paper making and so on [1-3]. But due to the poor aqueous solubility and instability of fragrance molecules, the fragrance use is limited [4-6]. Nowadays, fragrance encapsulation is emerging as a novel strategy to protect the small, weakly water-soluble aromatic compounds against rapid evaporation and oxidization [7-9]. So, it is possible to control the volatile substance release from the enclosed capsule as required.
Encapsulating a fragrance in nanocapsules is a simple process to fabricate polymeric nanocapsules in which the diffusion of the fragrant molecules is controlled by entrapping them into a polymeric matrix (carrier). Up to this point, current fragrance controlled release systems can be categorized into two groups, those that use a physical barrier system and a chemical barrier system [2], including envelope method, complex coacervation, situ/anionic polymerization, emulsion technology, physical mixing, interfacial polymerization, and spray drying/cooling technique [10-16]. D. P. Chattopadhyay et al. reported that the cyanuric chloride was used to utilize its reactivity which was grafted to β- cyclodextrin to generate cavities to load various fragrances in it. Results clearly showed that attachment of β-cyclodextrin to cyanuric chloride whereas olfactometry analysis gave an idea of perfume retention onto the fabric [17]. Nanoencapsulation by the cyanuric chloride of the fragrance appears to be an effective technique for fragrance encapsulation, however, the cyanuric chloride is toxic to humans.
Fragrance controlled release technology for personal care and natural food additives should not only provide fragrance prolongation but also possess non-toxicity and biocompatibility [6]. In particular, researchers have focused on natural encapsulating materials because of the desirable properties of biocompatibility and biodegradability [18-23]. The traditional natural encapsulating materials include chitosan, sodium alginate and glutin. W. Supason et al. used non-toxic, good biocompatibility ethyl cellulose (EC), a mixed polymer hydroxypropylmethylcellulose and polyvinyl alcohol entrapped perfume [13]. Interestingly, Supason P. Wanichwecharungruang et al. demonstrated the fabrication of long lasting fragrance controlled release systems which contain both chemical and physical barriers, based on the biocompatible biodegradable non-toxic chitosan polymer [24].
Although a large amount of methods have been reported to improve the slow release of fragrance to achieve stability and durability of fragrance, but each method has its specific limitations, or the production process is high cost, or the industrial conditions are difficult to achieve, or the physical and chemical properties are interaction between wall material and the fragrance, as well as flavor wall material and the core material. The ideal fragrance carrier system with simple process is still being explored, particularly flavor systems for applications in the household and natural food additives. And the safe, non-toxic, biocompatible flavor delivery system is the most demanding applications system.
In this investigation, soya lecithin and DSPE-PEG(2000) as a polymeric shell, PLGA as a core material, novel biocompatible nanocapsules (soya lecithin/DSPE-PEG(2000)/PLGA) loaded with lily fragrance (LF) were prepared using solvent displacement (ethanol displaced by water) method. Having super hydrophobic and desirable properties of biocompatibility and biodegradability, PLGA is a copolymer of lactic acid and glycolic acid, as flavor microcapsules core, which can interact with oily fragrance. Soya lecithin and DSPE-PEG(2000) are amphiphilic molecules as micro flavor shell of the capsule, hydrophilic part contacting with water, hydrophobic part interaction with PLGA. Among them, soya lecithin is extracted from natural soy liposome, biocompatibility and lowing prices, PLGA and DSPE-PEG(2000) are approved polymers by the US Food and Drug Administration (FDA). So the use of soyba lecithin/DSPE-PEG (2000)/PLGA encapsulated lily fragrance is safety and low toxicity, and can effectively improve the flavor volatile stability.
The optimal conditions of the preparation of the LF-NPs were confirmed by a series of single factor experiments and orthogonal experimental process. The influence of reaction conditions, such as the amount of molar ratio of lecithin, the molar ratio of PLGA and temperature, on the particle size, encapsulation efficiency and particle size distribution (PDI) of nanoparticles was investigated in detail for the best preparation conditions. The impact of the novel biocompatible nanocapsules on the human cell was studied by a range of in vitro experiments by using the MTT assay. These experiments offer an insight into the biological impact of LF-NPs on the human.
A representation of the solvent displacement method for the preparation of LF-NPs was showed in Fig. 1. Amongst polymers used in the blend (soya lecithin, PLGA and DSPE-PEG(2000)), wellaccepted nontoxic biocompatible PLGA is high hydrophobicity properties, soya lecithin is amphiphilic molecules which is extracted from natural soy liposome, biocompatibility and lowing prices. At first, the oil-soluble polymer and the lily fragrance were dissolved in an organic solvent, and then the lily fragrance was slowly added an aqueous ethanol solution of lecithin molecules to the system. Therefore, the LF-NPs were obtained through selfassembly method. The oil-soluble lily fragrance was entrapped in the hydrophobic layer cavity, greatly improving the stability of flavor lily fragrance and product quality. All the obtained particles showed readily dispersible in water formed a colloidal solution, the appearance was clear and transparent appearance, no morphological change, and the method is simple and low cost.

|
Download:
|
| Fig. 1. A representation of the solvent displacement method for the preparation of LF-NPs. | |
The temperature has a paramount importance in controlling the variety of reaction products. In order to select of suitable temperature for the LF-NPs, the influence of temperature on the particle size of LF-NPs was evaluated. Fig. 2A shows the influence of the temperature on the mean size of the LF-NPs with the temperature of 25 ℃, 30 ℃, 35 ℃ and 40 ℃, respectively. With increasing the temperature, the size of the LF-NPs increased. The smaller size of nanoparticles can often serve to achieve therapeutic goals where micro-sized or larger fragrance-delivery systems fail.

|
Download:
|
| Fig. 2. (A) Effect of the temperature on the mean diameter of the LF-NPs. (B) Effect of the molar ratio of lecithin on the mean diameter of the LF-NPs. (C) Effect of the molar ratio of PLGA on the mean diameter of the LF-NPs. | |
Generally, a high temperature led to the enhancement of the particle size during emulsion polymerization. With the high agitation rates, the emulsion particles consequently broke and reagglomerated, and also, the stability of the emulsions might have decreased, so the size of the LF-NPs improved. In the end, among the four kinds of temperatures, only temperature with 25 ℃ facilitated to prepare well-structured nanoparticles, so we chose this temperature as the optimum temperature.
The effect of the molar ratio of lecithin on the mean diameter of LF-NPs was shown in Fig. 2B. The mean diameter of LF-NPs firstly decreased and then increased with the increase in lecithin concentration, meanwhile, the content of lecithin had not obviously influenced the size distribution of the nanocapsules. The mean diameter of the nanocapsules was about 140 nm when the molar ratio of lecithin/DSPE-PEG(2000)/PLGA was 8.5:1.5:0.6. With increasing amounts of lecithin, other three fragrance systems the size became a little large, and the solution was turbid, not suitable for the application. Combination of the above analysis, we selected the sample with the molar ratio of 8.5:1.5:0.6 as the optimum amount of lecithin.
According to the analysis above, we fixed lecithin and DSPE-PEG (2000) molar ratio of 8.5:1.5, changed the molar ratio of PLGA. The content of PLGA also has a significant effect on the size of the nanocapsules. As can be seen from Fig. 2C, the mean size of the nanocapsules firstly decreased and then increased as the concentration of PLGA increased. When the molar ratio of PLGA was 2.0, the average diameter of the nanocapsules was 226.1 nm; however, the average diameter decreased to 100 nm as the PLGA increased to 7.0. An increase in PLGA can produce smaller nanocapsules with a more homogeneous size distribution. When more PLGA was added to the system, during the reaction process, lily fragrance monomers solubilized into the micelles were polymerized to encapsulate lily fragrance via free-radical polymerization so that more PLGA as the core of the nanocapsule lead to the improvement of the LF-NPs size.
The TGA analysis, based upon the weight loss from the solid sample, where the reduction of the amount of fragrances remaining in the samples corresponds to the fragrance release behavior of the sample, was shown in Fig. 3 and Figs. S2–S4 (Supporting information). Fig. 3 displayed the TGA curves of the nanoparticles with different contents of lecithin/DSPE-PEG(2000)/ PLGA, respectively. The TGA indicated that unencapsulated fragrance gave the most significant burst at the beginning 50 ℃, when the temperature up to 200 ℃, the average loss of fragrance components was 96.7%. Nevertheless, the loss of encapsulated fragrances burst at 220 ℃. The thermal stability of fragrance encapsulated in LF-NPs was improved. However, the stability behaviors of all encapsulated fragrances could be improved by this polymeric encapsulation with different extents. Then comparing five encapsulated fragrances groups of samples, the encapsulation efficiency of nanocapsule was highest, with loading ratio of 21.1%, when the molar ratio of lecithin:DSPE-PEG(2000):PLGA:was 8.5:1.5:7.0. The efficiency of the fragrance encapsulation depended on the miniemulsion polymerization process with a homogeneous mixture of the dispersed phase consisting of the lecithin, DSPEPEG(2000) and the PLGA.

|
Download:
|
| Fig. 3. TGA result (F: Free LF; A: NPs; B: LF-NPs). | |
Interactions between fragrance molecules and polymer matrix could delay the volatilization of the fragrance. It was noticed that after being encapsulated, the vapor pressure and boiling point of the fragrance were no longer the major factors determining the release rate. Rather the release characteristics are likely to be governed by the ability of the fragrance to diffuse through the polymer matrix, and fragrance molecules possess different chemical interactions with the polymer-blend-shell, and thus diffusion rates.
When visualizing the LF-NPs by TEM, the samples show a homogeneous structure (Fig. S1 in Supporting information). The TEM micrographs in Fig. S1 clearly illustrated that LF-NPs were spherical particles. Their scattered homogeneous size (the average diameters keep less than 100 nm) confirmed the results from the dynamic light scattering (DLS) analysis, and compared with control sample, the fragrance nanocapsules had little change in the particle size. Therefore, the successful formation of nanocapsules was confirmed.
The evaluation of the toxicity and compatibility of a new system are necessary to investigate. Fig. 4 displayed the cell viability of the nanocapsules by an MTT assay using HEK293 cells. There was no significant cell death observed when the cells were treated with different concentrations of the nanocapsules after 2 days. In addition, with increasing concentration, there was no variation of cell viability, which can be explained by the non-toxic, biocompatible and biodegradable biopolymers of lecithin, DSPE-PEG (2000) and PLGA. Results of this study provide applicable information considering the eco-friendly bio-material of lecithin, DSPE-PEG(2000) and PLGA. Since the studied lavender extracts did not decrease cell viability, their use in cutaneous formulations is not expected to be limited by safety concerns.

|
Download:
|
| Fig. 4. Effect of LF-NPs extracts on the viability of human HEK293 cells. | |
The strong volatility of LF resulted in its short useful life, which severely limits the applications of LF products. So the release profiles of free LF and LF-NPs were measured. As shown in Fig. 5, after 24 h, 42.47% of free LF was released and only 6.20% of LF was released from LF-NPs. This result indicated that LF-NPs could significantly prolong the release time of lily fragrance.
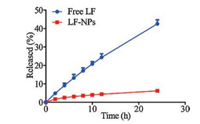
|
Download:
|
| Fig. 5. The release profiles of free LF and LF-NPs. The mean ± SD is shown (n = 3). | |
In summary, novel biocompatible nanocapsules encapsulated lily fragrance (LF-NPs) were development. The mean diameter of LF-NPs was about 100 nm and the encapsulation of lily fragrance was about 21.9%. Besides, due to the non-toxic ingredients of nanocapsules, LF-NPs offer biocompatibility. In addition, with the sustained release of lily fragrance, the LF-NPs have long used life. And preparation of LF-NPs is simple operation and low cost. Therefore, the LF-NPs are expect to have many potential applications to our daily life, such as cosmetic decorative, food industry, antibacterial, medical industry, tobacco industry, textile industry, home life, and so on.
AcknowledgmentThis work was financially supported by the National High Technology Research and Development Program (No. 2016YFA0200303), the Beijing Natural Science Foundation (No. 2164071), the National Natural Science Foundation of China (Nos. 31522023, 31771095, and 51573188), the Beijing Municipal Science & Technology Commission (No. Z161100002616015). All procedures involving experimental animals were performed in accordance with protocols approved by the Institutional Animals Care and Use Committee of Peking University.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.09.008.
| [1] |
S. Fukumoto, E. Sawasaki, S. Okuyama, Y. Miyake, H. Yokogoshi, Nutr. Neurosci. 9 (2006) 73-80. DOI:10.1080/10284150600573660 |
| [2] |
A. Herrmann, Angew. Chem. Int. Ed. Engl. 46 (2007) 5836-5863. |
| [3] |
T. Morita, K. Jinno, H. Kawagishi, et al., J. Agric. Food Chem. 51 (2003) 1560-1565. DOI:10.1021/jf020946n |
| [4] |
M. Matura, M. Sköld, A. Börje, et al., Contact Derm. 55 (2006) 274-279. DOI:10.1111/cod.2006.55.issue-5 |
| [5] |
Z. Wen, X.K. You, L.Z. Jiang, et al., Flavour Fragr. J. 26 (2011) 27-33. DOI:10.1002/ffj.v26.1 |
| [6] |
A. Madene, M. Jacquot, J. Scher, S. Desobry, Food Sci. Technol. 4 (2006) 1-21. |
| [7] |
Z.G. Lu, T.L. Zhang, J. Shen, et al., J. Biomed. Nanotechnol. 14 (2018) 1578-1589. DOI:10.1166/jbn.2018.2607 |
| [8] |
J. Shen, Z.G. Lu, T.L. Zhang, et al., J. Biomed. Nanotechnol. 14 (2018) 1556-1567. DOI:10.1166/jbn.2018.2608 |
| [9] |
Z.G. Lu, Y.C. Zheng, T.L. Zhang, et al., J. Biomed. Nanotechnol. 14 (2018) 1675-1687. DOI:10.1166/jbn.2018.2611 |
| [10] |
Y. Lv, F. Yang, X.Y. Li, X.M. Zhang, S. Abbas, Food Hydrocoll. 35 (2014) 305-314. DOI:10.1016/j.foodhyd.2013.06.003 |
| [11] |
M.S. María, E. Germán, M. Patricia, J. Ind. Text. 40 (2010) 13-32. DOI:10.1177/1528083709350184 |
| [12] |
J. Hu, Z.B. Xiao, R.J. Zhou, Chin. J. Chem. Eng. 19 (2011) 523-528. DOI:10.1016/S1004-9541(11)60016-5 |
| [13] |
A. Sansukcharearnpon, S. Wanichwecharungruang, N. Leepipatpaiboon, T. Kerdcharoen, S. Arayachukeat, Int. J. Pharm. 391 (2010) 267-273. DOI:10.1016/j.ijpharm.2010.02.020 |
| [14] |
J. Hu, W.J. Deng, L.Q. Liu, Z.B. Xiao, J. Appl. Polym. Sci. (2014) 40182. |
| [15] |
A. Esmaeili, S. Niknam, Flavour Fragr. J. 28 (2013) 309-315. DOI:10.1002/ffj.v28.5 |
| [16] |
J. Hu, Z.B. Xiao, R.J. Zhou, et al., Flavour Fragr. J. 26 (2011) 162-173. DOI:10.1002/ffj.v26.3 |
| [17] |
D.P. Chattopadhyay, J. Shweta, TLIST 2 (2013) 62-70. |
| [18] |
B. Hosseinkhani, C. Callewaert, N. Vanbeveren, N. Boon, Biotechnology 32 (2015) 40-46. |
| [19] |
F. Nugier, J.N. Colin, M. Aymard, M. Langlois, J. Med. Virol. 36 (1992) 1-12. |
| [20] |
D. Valenti, A. De Logu, G. Loy, et al., J. Liposome Res. 11 (2001) 73-90. DOI:10.1081/LPR-100103171 |
| [21] |
S. Tan, X. Li, Y. Guo, Z. Zhang, Nanoscale 5 (2013) 860-872. DOI:10.1039/c2nr32880a |
| [22] |
U. Paiphansiri, P. Tangboriboonrat, K. Landfester, Macromol. Biosci. 6 (2006) 33-40. |
| [23] |
S.L. Tirilly, C. Tregouet, S. Bône, et al., ACS Macro Lett. 4 (2015) 25-29. DOI:10.1021/mz5005772 |
| [24] |
T. Tree-udom, S.P. Wanichwecharungruang, J. Seemork, S. Arayachukeat, Carbohyd. Polym 86 (2011) 1602-1609. DOI:10.1016/j.carbpol.2011.06.074 |
 2019, Vol. 30
2019, Vol. 30 

