b State Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, China
Pillar[n]arenes [1-5] have been a promising class of macrocycles constituted by 1, 4-dimethoxylbenzene rings linked by methylene bridges with a symmetric, rigid and tunable structure [6-12]. Several practical applications, such as host-guest chemistry [13-18], sensors [19-29], molecular machines [30-33], selfassemblies [34-36], drug delivery [37-40] and hybrid organicinorganic materials [41, 42] have been reported. These applications were mostly based on the functionalization of pillar[n]arenes by functionalizing the rims and controlling their solubility in water and organic solvents. The commonly used methodologies for the functionalization of pillar[5]arene involves one or multiple monomers that cyclize in the presence of a Brønsted acid or Lewis acid catalyst, and post synthetic modifications can lead to mono-, bis- and multi-substituted pillar[5]arene.
The first fully substituted pillar[5]arene was reported by Ogoshi [43], and the portfolio of their functionalization was extended to the conception of artificial transmembrane proton channel [44], stabilization of gold nanoparticles [45], construction of particular molecular machines and so on. Mono-substituted elegant methodology of synthetic post-modification involving the oxidation of a ring followed by a regioselective reduction pillar[5]arene was reported by Cao's group by adopting an ester group into a phenolic motif which was subsequently substituted [46]. The methodology to the direct construction of monoester-substituted pillar[5]arene has also been poorly exploited by direct cyclization with 2 monomers [47, 48]. These unique monoester-substituted pillar[5] arene can be used not only for molecular recognition but also for the construction of stable [1]rotaxanes with potential catalytic capacities, and multi-pillar[5]arene metallacycles [49].
Due to their wide applications, easier methods to produce bisester-functionalized copillar[5]arene are still on demand. Based on our previous work [47], we found that the precursor, diethyl 2, 2'-(1, 4-phenylenebis(oxy))diacetate (M), was appropriate for the construction of bisester-functionalized copillar[5]arene (BECP5A), which is more efficient and economic than the reported synthetic methodology that at least required four steps (with an overall yield of 28%), as shown in Scheme 1. In this work, BECP5A was deeply investigated both in solution and in solid state with the aid of 2D NMR spectrum and X-ray crystal diffraction.

|
Download:
|
| Scheme 1. Synthetic methodologies of BECP5A by post-modification and premodification: Route Ⅰ (blue) and Route Ⅱ (red). | |
Following another synthetic route (Route Ⅱ), hydroquinone was reacted with ethyl bromoacetate leading to the ester containing precursor M, then the condition to synthesize BECP5A was optimized, among which the most suitable one was described as: 1, 4-dimethoxybenzene (30.0 mmol) in dichloromethane (250.0 mL) was added paraformaldehyde (109.5 mmol) and M (6.0 mmol), then the suspension was stirred at 0 ℃ for 30 min, then boron trifluoride diethyl etherate (42.5 mmol) was added, and the mixture was stirred at 0 ℃ for 90 min. The final compound was obtained with yield up to 20%.
The obtained compound was firstly analyzed by electrospray ionization mass spectra (Figs. S9 and S10 in Supporting information) which gave m/z calcd. for C51H59O14+ [M+H]+: 895.390; found: 895.391. Calcd. for C51H62NO14+ [M + NH4]+: 912.416; found: 912.415. Calcd. for C51H58NaO14+ [M + Na]+: 917.372; found: 917.370. Calcd. for C51H58KO14+ [M + K]+: 933.346; found: 933.342. Compound BECP5A was characterized by 1H NMR and 13C NMR spectra (Figs. S5-S8 in Supporting information). When CDCl3 was used as the solvent, a strong sharp triplex peak appeared in the range of high-field chemical value below 0 ppm, which indicated that BECP5A is potentially self-included in chloroform. To further investigate this phenomenon, the comparison of the NMR spectra of M and BECP5A was done and shown in Fig. 1. From Fig. 1a, HA' and HB' were up-field shifted to δ 3.13 and δ -0.26, compared to δ 4.26 and δ 1.29, respectively from M. The ΔδH of HA and HB were found to be 1.13 ppm and 1.55 ppm, respectively. From Fig. 1b, 13C chemical shifts of CA' and CB' were upfield shifted to δ 61.2 and δ 12.6, compared to δ 61.5 and δ 14.4, respectively. The ΔδC of CA and CB were found to be 0.3 ppm and 1.8 ppm. These obvious chemical shift changes were further supported by Nuclear Overhauser Effect Spectroscopy (NOESY) where the proximity between the protons has to be below 5 Å to see the spatial correlations. From Fig. 2, it is clear that HA' and HB' are all close to the methylene bridges or methoxy groups HC, D and benzylic hydrogens, HAr. However, the difference of chemical shifts Δδ value were found to be low when DMSO-d6 was used as the solvent. To further give a convinced evidence, one equivalent of malononitrile was added to a solution of BECP5A in CDCl3, and an obvious downfield shift of the ethyl groups to 3.55 ppm for HA' and 0.29 ppm for HB' were observed by displacement (Fig. S14 in Supporting information). We can conclude that in solution, BECP5A have a symmetric and self-included conformation in chloroform where the cavity hosts the pair of ester groups which is not the case in DMSO.

|
Download:
|
| Fig. 1. 1H NMR (a) and partial 13C NMR spectra (b) of M and BECP5A in CDCl3. | |

|
Download:
|
| Fig. 2. Partial NOESY spectrum (600 MHz, CDCl3, 298 K) of BECP5A. | |
Based on the above conclusions, we were wondering if the cavity of pillar[5]arene can host a pair of ester groups in the solid state. For this purpose, the single crystal was grown by slow evaporation of a solution in chloroform/methanol. Although the difficulties of obtain the crystal structure, we finally solved the crystal structure as shown in Figs. 3a-c. The real structure of BECP5A exists with only one side of ester group hosted in the cavity of pillar[5]arene excluding the other one outer the cavity. From Figs. 3a and b, the intramolecular C-H…π interactions and C- H…O interactions play a vital role in the stabilization of this selfincluded conformation (Fig. S16 in Supporting information). We found from the crystal structure two prochiral conformations (pS and pR) contained in a unit cell which are shown in Fig. 3c.
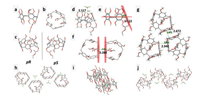
|
Download:
|
| Fig. 3. X-ray crystal structure of a selected pS conformation of BECP5A: a) side view, b) top view, c) pR and pS conformations, d) C-H…O interaction between chloroform and carboxylate oxygen of BECP5A, e) dihedral angle between bisester-functionalized benzene ring and the plane of copillar[5]arene, f) intermolecular C-H…π and g) π…π interactions of BECP5A. Packing mode of BECP5A along h) [100], i) [010], j) [001] directions. Parts of hydrogen atoms have been omitted for clarity. C: grey, O: red. Cl: green. | |
Additional investigations of the crystal structure are shown in Figs. 3d-g. From Fig. 3d, we noticed strong C-H…O interactions between the hydrogens belonging to chloroform and oxygen of one carboxylate group, which contributes to the stabilization of the asymmetrical structure of BECP5A. Figs. 3f-g show that there exists intermolecular C-H…π and π…π interactions of BECP5A. The dihedral angle between bisester-functionalized benzene ring and the plane of copillar[5]arene in BECP5A was measured to 67.22°, as shown in Fig. 3e.
The ratio of CDCl3/CD3OD was fluctuated to see the effects on the self-inclusion (Fig. S14 in Supporting information), an increased ratio of methanol led to the removal of the ester groups from the cavity proving that the polarity of methanol breaks the stabilizing interactions in favor of the self-inclusion.
In conclusion, a bisester-substituted pillar[5]arene was synthesized by a one-pot reaction with yield up to 20% and fully characterized by 1H NMR, 13C NMR, NOESY, LC-MS and X-ray crystal structure. The properties of a such molecule were deeply investigated in the solution state, showing that this molecule displayed a symmetric and self-included conformation with the two ester groups hosted in the cavity but in a partial self-included conformation in DMSO than chloroform due to the difference of polarity of solvents, and in the solid state, demonstrating that the compound has an asymmetrical structure with only one ester in the cavity of pillar[5]arene. Further applications of BECP5A has been explored in our lab and is an interesting intermediate for the construction of mechanically interlocked structure or polymers.
AcknowledgmentWe thank the JLU Cultivation Fund for the National Science Fund for Distinguished Young Scholars, and NMAC, College of Chemistry at Jilin University for financial support.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.12.015.
| [1] |
T. Ogoshi, S. Kanai, S. Fujinami, T.-a. Yamagishi, Y. Nakamoto, J. Am. Chem. Soc. 130 (2008) 5022-5023. DOI:10.1021/ja711260m |
| [2] |
P.J. Cragg, K. Sharma, Chem. Soc. Rev. 41 (2012) 597-607. DOI:10.1039/C1CS15164A |
| [3] |
T. Ogoshi, T.-a. Yamagishi, Y. Nakamoto, Chem. Rev. 116 (2016) 7937-8002. DOI:10.1021/acs.chemrev.5b00765 |
| [4] |
M. Xue, Y. Yang, X. Chi, Z. Zhang, F. Huang, Acc. Chem. Res. 45 (2012) 1294-1308. DOI:10.1021/ar2003418 |
| [5] |
T. Kakuta, T.-a. Yamagishi, T. Ogoshi, Acc. Chem. Res. 51 (2018) 1656-1666. DOI:10.1021/acs.accounts.8b00157 |
| [6] |
X.B. Hu, Z. Chen, G. Tang, J.L. Hou, Z.T. Li, J. Am. Chem. Soc. 134 (2012) 8384-8387. DOI:10.1021/ja302292c |
| [7] |
D.R. Cao, Y.H. Kou, J.Q. Liang, et al., Angew. Chem. 121 (2009) 9901-9903. DOI:10.1002/ange.200904765 |
| [8] |
J.F. Stoddart, N.L. Strutt, R.S. Forgan, J.M. Spruell, Y.Y. Botros, J. Am. Chem. Soc. 133 (2011) 5668-5671. DOI:10.1021/ja111418j |
| [9] |
Z.B. Zhang, B.Y. Xia, C.Y. Han, Y.H. Yu, F.H. Huang, Org. Lett. 12 (2010) 3285-3287. DOI:10.1021/ol100883k |
| [10] |
C.J. Li, K. Han, J. Li, et al., Org. Lett. 14 (2012) 42-45. DOI:10.1021/ol2027834 |
| [11] |
J.R. Wu, C.Y. Wang, Y.C. Tao, et al., Eur. J. Org. Chem. 2018 (2018) 1321-1325. DOI:10.1002/ejoc.v2018.11 |
| [12] |
J.R. Wu, A.U. Mu, B. Li, et al., Angew. Chem. Int. Ed. 57 (2018) 9853-9858. DOI:10.1002/anie.201805980 |
| [13] |
X.B. Hu, L. Chen, W. Si, Y. Yu, J.L. Hou, Chem. Commun. 47 (2011) 4694-4696. DOI:10.1039/c1cc10633c |
| [14] |
Y.J. Ma, X.F. Ji, F. Xiang, et al., Chem. Commun. 47 (2011) 12340-12342. DOI:10.1039/c1cc15660h |
| [15] |
T. Ogoshi, J. Incl. Phenom. Macrocycl. Chem. 72 (2012) 247-262. DOI:10.1007/s10847-011-0027-2 |
| [16] |
F.H. Huang, Z.B. Zhang, Y. Luo, et al., Angew. Chem. 123 (2011) 1433-1437. DOI:10.1002/ange.v123.6 |
| [17] |
Z.B. Zhang, G.C. Yu, C.Y. Han, et al., Org. Lett. 13 (2011) 4818-4821. DOI:10.1021/ol2018938 |
| [18] |
X.Y. Shu, S.H. Chen, J. Li, et al., Chem. Commun. 48 (2012) 2967-2969. DOI:10.1039/c2cc00153e |
| [19] |
J.H. Bi, X.F. Zeng, D.M. Tian, H.B. Li, Org. Lett. 18 (2016) 1092-1095. DOI:10.1021/acs.orglett.6b00097 |
| [20] |
Y.C. Chang, K. Yang, P. Wei, et al., Angew. Chem. 53 (2014) 13126-13130. DOI:10.1002/anie.201407272 |
| [21] |
P. Wang, Y. Yao, M. Xue, Chem. Commun. 50 (2014) 5064-5067. DOI:10.1039/C4CC01403K |
| [22] |
X. Wu, Y. Li, C. Lin, X.Y. Hu, L.Y. Wang, Chem. Commun. 51 (2015) 6832-6835. DOI:10.1039/C5CC01393C |
| [23] |
Q.F. Yao, B.Z. Lv, C.D. Ji, Y. Cai, M.Z. Yin, ACS Appl. Mater. Interfaces 9 (2017) 36320-36326. DOI:10.1021/acsami.7b12063 |
| [24] |
G.C. Yu, C.Y. Han, Z.B. Zhang, et al., J. Am. Chem. Soc. 134 (2012) 8711-8717. DOI:10.1021/ja302998q |
| [25] |
F. Zhang, J.K. Ma, Y. Sun, et al., Chem. Sci. 7 (2016) 3227-3233. DOI:10.1039/C5SC04726A |
| [26] |
H.T.Z. Zhu, L.Q. Shangguan, B.B. Shi, G.C. Yu, F.H. Huang, Mater. Chem. Front. 2 (2018) 2152-2174. DOI:10.1039/C8QM00314A |
| [27] |
J.F. Chen, Q. Lin, Y.M. Zhang, H. Yao, T.B. Wei, Chem. Commun. 53 (2017) 13296-13311. DOI:10.1039/C7CC08365C |
| [28] |
X. Li, Z. Li, Y.W. Yang, Adv. Mater. 30 (2018) 1800177. DOI:10.1002/adma.v30.20 |
| [29] |
X.Y. Jin, N. Song, X. Wang, et al., Sci. Rep. 8 (2018) 4035. DOI:10.1038/s41598-018-22446-y |
| [30] |
T. Ogoshi, D. Yamafuji, T.-a. Yamagishi, A.M. Brouwer, Chem. Commun. 49 (2013) 5468-5470. DOI:10.1039/c3cc42612b |
| [31] |
X. Wu, M.F. Ni, W. Xia, X.Y. Hu, L.Y. Wang, Org. Chem. Front. 2 (2015) 1013-1017. DOI:10.1039/C5QO00159E |
| [32] |
X.Z. Yan, P.F. Wei, Z.T. Li, et al., Chem. Commun. 49 (2013) 2512-2514. DOI:10.1039/c3cc40474a |
| [33] |
X.Z. Yan, B. Zheng, F.H. Huang, Polym. Chem. 4 (2013) 2395-2399. DOI:10.1039/c3py00060e |
| [34] |
C.J. Li, X.Y. Shu, J. Li, et al., Org. Lett. 14 (2012) 4126-4129. DOI:10.1021/ol301757q |
| [35] |
X. Wu, Y. Yu, L. Gao, X.Y. Hu, L.Y. Wang, Org. Chem. Front. 3 (2016) 966-970. DOI:10.1039/C6QO00197A |
| [36] |
H.C. Zhang, Y.L. Zhao, Chem. Eur. J. 19 (2013) 16862-16879. DOI:10.1002/chem.v19.50 |
| [37] |
L.Y. Gao, B. Zheng, W. Chen, C.A. Schalley, Chem. Commun. 51 (2015) 14901-14904. DOI:10.1039/C5CC06207A |
| [38] |
K. Yang, Y.C. Chang, J. Wen, et al., Chem. Mater. 28 (2016) 1990-1993. DOI:10.1021/acs.chemmater.6b00696 |
| [39] |
Y.J. Zhou, K.C. Jie, F.H. Huang, Chem. Commun. 53 (2017) 8364-8367. DOI:10.1039/C7CC04779G |
| [40] |
X.Y. Shu, K.D. Xu, D.B. Hou, C.J. Li, Isr. J. Chem. 58 (2018) 1194-1204. DOI:10.1002/ijch.201800013 |
| [41] |
J.F. Chen, X. Liu, J.F. Ma, et al., Soft Matter 13 (2017) 5214-5218. DOI:10.1039/C7SM01118K |
| [42] |
N. Song, T. Kakuta, T.-a. Yamagishi, Y.W. Yang, T. Ogoshi, Chem 4 (2018) 2029-2053. DOI:10.1016/j.chempr.2018.05.015 |
| [43] |
T. Ogoshi, M. Hashizume, T.-a. Yamagishi, Y. Nakamoto, Chem. Commun. 46 (2010) 3708-3710. DOI:10.1039/c0cc00348d |
| [44] |
W. Si, L. Chen, X.B. Hu, et al., Angew. Chem. 123 (2011) 12772-12776. DOI:10.1002/ange.201106857 |
| [45] |
H. Li, D.X. Chen, Y.L. Sun, et al., J. Am. Chem. Soc. 135 (2013) 1570-1576. DOI:10.1021/ja3115168 |
| [46] |
Y. Chen, D. Cao, L. Wang, et al., Chem. Eur. J. 19 (2013) 7064-7070. DOI:10.1002/chem.v19.22 |
| [47] |
X.S. Du, C.Y. Wang, Q. Jia, et al., Chem. Commun. 53 (2017) 5326-5329. DOI:10.1039/C7CC02364B |
| [48] |
Y. Han, G.F. Huo, J. Sun, et al., Sci. Rep. 6 (2016) 28748. DOI:10.1038/srep28748 |
| [49] |
Z.Y. Li, Y. Zhang, C.W. Zhang, et al., J. Am. Chem. Soc. 136 (2014) 8577-8589. DOI:10.1021/ja413047r |
 2019, Vol. 30
2019, Vol. 30 

