b Wuhan National Laboratory for Optoelectronics, Huazhong University of Science and Technology, Wuhan 430074, China;
c No. 1 Middle School Affiliated to Central China Normal University, Wuhan 430075, China
Hydrogen is considered as an ideal alternative energy carrier in the future to address the concerns derived from rapid growth of energy consumption, as well as the associated environmental issues [1-3]. However, safe and efficient storage of hydrogen has been still regarded as one of the key issues for the upcoming widespread of "hydrogen economy" [4-10]. Nowadays, enormous efforts have been devoted to developing efficient hydrogen storage materials, includingmetalhydrides, absorptionmaterials, and chemicalhydrides[11-14]. Among them, hydrous hydrazine (N2H4·H2O) with high hydrogen content (8.0 wt%) has been regarded as a promising candidate for chemical hydrogen storage application due to its high stability, easy recharging ability and potentiality to utilize the existing liquid-based distribution infrastructure [15-17]. Hydrous hydrazine can be decomposed following two basic principle pathways, while the undesired reaction pathway (Eq. 2) should be avoided for the reason that ammonia is a fatal poison to fuel cell catalysts [18]. Therefore, searching for highly efficient nanocatalysts with high hydrogen selectivity toward dehydrogenation of hydrazine is extremely desirable for its practical application.
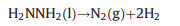
|
(1) |
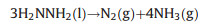
|
(2) |
Among various reported catalysts for catalytic dehydrogenation of hydrazine, NiPt-based nanocatalysts have received considerable interests due to their high catalytic performances and hydrogen selectivity [19-22]. For instance, Xu's group first reported bimetallic Ni-Pt nanocatalysts for selective decomposition of hydrazine in alkaline solution with a TOF value of 7.9 h–1 at room temperature [23]. Follow that, Jiang's group reported NiPt NPs supported on Ce2O3 for catalyzing hydrazine dehydrogenation with a TOF value of 28.1 h–1 at 298 K [24]. Wang's group reported core-shell Ni@NiPt NPs supported on La2O3 for catalyzing hydrazine dehydrogenation with a TOF value of 312 h–1 at 323 K [25]. Recently, our group reported the synthesis of metal-organic framework (MOF)-encapsulated NiPt NPs and their enhanced catalytic performance for hydrogen generation from hydrous hydrazine with a TOF value of 375.1 h–1 at 323 K [26]. Despite remarkable progresses have been achieved, searching for highlyefficient catalysts with excellent catalytic performances and high hydrogen selectivity for the dehydrogenation of hydrazine, especially at room temperature, still remains great challenging.
It has been reported that CeO2 nanospheres can be used as ideal substrate to load nanocatalysts, due to its intrinsic property such as high stability, high specific surface area, and redox properties, which are regarded as key factors for boosting the catalytic performance [27-31]. Following this strategy, herein, we reported a simple in situ co-reduction method to synthesize NiPt NPs loaded on CeO2 nanospheres, and their excellent catalytic performance toward hydrogen generation from alkaline solution of hydrazine at room temperature. As expected, by taking the advantage of strong support-metal interaction and synergistic electronic effect between NiPt and CeO2, the obtained Ni5Pt5-CeO2 catalyst exhibits remarkable catalytic activity and 100% hydrogen selectivity for dehydrogenation of hydrous hydrazine, with the turnover frequency (TOF) value of 416 h-1 at room temperature, which is higher than most of the reported catalysts.
CeO2 nanospheres were first synthesized via a hydrothermal method as reported by Singha et al. [32]. Then, NiPt nanoparticles with different molar ratios were successfully loaded on the assynthesized CeO2 nanospheres through a simple in situ coreduction method and used for catalyzing the decomposition of hydrazine in alkaline solution (0.75 mol/L NaOH) at room temperature (More details can be found in experimental section in Supporting information). As shown in the Fig. 1a, Ni-CeO2 exhibits almost no catalytic activity. Surprisingly, when alloyed with Pt, the obtained NiPt-CeO2 catalysts with different ratios of metal exhibit much enhanced catalytic activities and hydrogen selectivity. Particularly, when the molar ratios of Ni and Pt is 1:1, the obtained Ni5Pt5-CeO2 exhibits the highest catalytic activity and 100% hydrogen selectivity with a TOF value of 416 h-1, which is higher than most of the reported catalysts as shown in Table S1 (Supporting information). Meanwhile extremely low catalytic activity is also observed for Pt-CeO2 catalyst, highlighting the optimized molar ratio of Ni and Pt plays a pivotal role in enhancing the catalytic performance for hydrogen generation from alkaline solution of hydrazine. In addition, to study the key effect of CeO2, the catalysts with optimized molar ratio of Ni and Pt supported on other supports, such as XC-72, reduced graphene oxide (rGO), or without support material were also prepared and tested for dehydrogenation of hydrazine. As shown in the Fig. 1b, their catalytic performances are all inferior to that of Ni5Pt5-CeO2, suggesting the key factor of CeO2 nanospheres in facilitating catalytic performance.

|
Download:
|
| Fig. 1. (a) Catalytic performance tests of NiPt-CeO2 catalyst with different molar ratios of metal in the presence of 0.75 mol/L NaOH at 298 K (a, b, c, d, e, f, and g, represent the Ni5Pt5-CeO2, Ni7Pt3-CeO2, Ni3Pt7-CeO2, Ni1Pt9-CeO2, Ni9Pt1-CeO2, Ni-CeO2, Pt-CeO2. (b) Catalytic performance of Ni5Pt5 on different supporter toward the decomposition of hydrazine at 298 K. (c) Catalytic performance of Ni5Pt5-CeO2 in alkaline solution with different concentrations of NaOH at 298 K. (d) Durability tests of Ni5Pt5-CeO2 toward the decomposition of hydrazine at 298 K. | |
It has been reported that the alkaline environment could facilitate the process of hydrazine decomposition [33]. The catalytic activity of as-synthesized Ni5Pt5-CeO2 was tested under different concentration of NaOH for hydrazine dehydrogenation to study the effect of OH- species. As shown in the Fig. 1c, without NaOH, only less than 0.5 equiv. of gas is generated for more than 20 min, indicating that the incomplete dehydrogenation of N2H4·H2O with an extremely low reaction kinetics. Interestingly, after adding NaOH, the catalytic activity and hydrogen selectivity of catalysts are both increased significantly. Specially, when the concentration of the NaOH increased to 0.75 mol/L, the Ni5Pt5- CeO2 exhibits the highest catalytic performance. The possible reasons are as follows: First, OH- ions can boost the rate determining deprotonation step (N2H4→N2H3*+H*). Second, the generation process of NH3 can be inhibited in the alkaline condition [34]. However, further increased the amount of NaOH, resulting in the decreased on both the catalytic activity and hydrogen selectivity. Thus, an excess of OH- might interact with the surface of the catalyst and further affect the electron structure of the catalyst, resulting in the deactivation of the catalyst.
The durability of the Ni5Pt5-CeO2 catalyst was also tested by adding the same amount of hydrous hydrazine after the end of the previous reaction at room temperature. As can be seen clearly that even after five cycles of reactions, the Ni5Pt5-CeO2 catalyst still exhibits almost 100% hydrogen selectivity, with a slight decrease of catalytic activity (Fig. 1d). Furthermore, as shown in the Fig. 2a, the dehydrogenation of hydrazine were carried out at different temperatures (298–323 K) to determine the activation energy (Ea, see more details in Supporting information). As shown in Fig. 2b, the value of Ea for dehydrogenation of hydrazine catalyzed by Ni5Pt5-CeO2 catalyst is calculated to be 24.43 kJ/mol.

|
Download:
|
| Fig. 2. (a) Time plots for hydrogen generation toward the decomposition of hydrazine catalyzed by Ni5Pt5-CeO2 at temperatures ranging from 298 K to 323 K. (b) Arrhenius plot of lnk versus 1/T during the hydrazine decomposition over Ni5Pt5-CeO2. | |
The obtained NiPt-CeO2 catalysts with different molar ratios of Ni and Pt were characterized by powder X-ray diffraction (XRD). As shown in Fig. S1 (Supporting information), the diffraction peaks located at 28.5°, 33.1°, 47.5°, 56.3°, 59.1°, 69.4°, 76.7° and 79.1° belong to CeO2 are observed (PDF#34-0394). In addition, without addition of Ni, three sharp diffraction peaks appearing at 39.7°, 46.2° and 67.5° can be seen clearly from the XRD pattern of PtCeO2, which are assigned to Pt (PDF#04-0802). Similarly, without addition of Pt, almost no peaks can be observed from the XRD pattern of Ni-CeO2 (Fig. S2 in Supporting information). However, after calcined under the N2 (95%)/H2 (5%) at 550 ℃ for 2 h, the peaks at 44.5°, 51.8° and 76.4° corresponding to Ni (PDF#04-0850) can be observed clearly in the Ni-CeO2 (Fig. S3 in Supporting information). Particularly, for the NiPt-CeO2 catalysts, the diffraction peak located at around 40.6° can be seen clearly, which is between the fcc (111) diffraction peaks of Ni (PDF#04-0850) and Pt (PDF#04-0802). Moreover, the diffraction peak appearing at around 46.9° is also observed, which is between the fcc (200) diffraction peaks of Ni (PDF#04-0850) and Pt (PDF#04-0802). These results indicate the formation of NiPt alloy.
The Ni5Pt5-CeO2 and Ni5Pt5 catalysts were characterized by Xray photoelectron spectroscopy (XPS) to analyze the elementary compositions and electron interaction. As shown in Fig. 3a, the Ni 2p3/2 peak located at the binding energy of 852.4 eV for Ni5Pt5- CeO2 is belong to Ni0 [35]. In addition, Ni 2p3/2 peak appearing at the binding energy of 856.8 eV is assigned to the oxidized Ni. Meanwhile, the satellite peak located at the binding energy of 861.1 eV can be observed too. [36]. The formation of oxidized metals is probably own to the exposure to air during the process of catalysts preparation [37]. Meanwhile, Pt 4f7/2 peak appearing at the binding energy of 71.6 eV and Pt 4f5/2 peak appearing at the binding energy of 74.8 eV are both attributed to Pt0 (Fig. 3b) [38, 39]. Furthermore, as shown in Fig. 3c, both Ce3+ and Ce4+ are observed in the XPS spectrum of Ce 3d [40]. Specifically, the peaks of Ce 3d3/2 and Ce 3d5/2 appearing at the binding energies of 916.5 eV and 882.3 eV are assigned to Ce4+, which negatively shift of 0.2 eV and 0.4 eV respectively, comparing to those of pure ceric oxide [41-43]. The peaks of Ce 3d3/2 and Ce 3d5/2 appearing at the binding energies of 903.6 eV and 885.6 eV are assigned to Ce3+, which negatively shift of 0.3 eV and 0.2 eV respectively, comparing to those of pure ceric oxide [44, 45]. At the same time, it can be seen unambiguously that after loading NiPt nanoparticles on CeO2, the peaks of Ni 2p3/2 negatively shift of 0.2 eV, and Pt 4f5/2 positively shift of 0.6 eV, respectively, comparing to those of NiPt nanoparticles, which is probably due to the decrease of electron density and increase of d-band vacancies in the metal centers [46], indicating the electron transfer between NiPt and CeO2. All of the results indicate that there are strong electronic interaction between Ni, Pt, and Ce, which is beneficial to the enhancement of the catalytic performance toward dehydrogenation of hydrazine.

|
Download:
|
| Fig. 3. The XPS spectra of Ni 2p3/2 (a), Pt 4f (b) and Ce 3d (c) for the Ni5Pt5-CeO2. | |
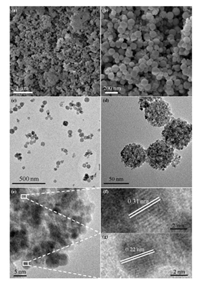
The morphology of the Ni5Pt5-CeO2 was characterized by scanning electron microscope (SEM) and transmission electron microscopy (TEM). As shown in Fig. S4 (Supporting information), well dispersed CeO2 nanospheres have been synthesized with the average size of 50 nm. After loading with NiPt NPs, the nanosphere morphology has been maintained well (Figs. 4a and b). The TEM images (Figs. 4c–e) indicate the Ni5Pt5-CeO2 display a highly dispersed spherical lacunose architecture, which could provide large amount of active sites and accelerate the adsorption of hydrazine, resulting in the enhancement of catalytic activity. Meanwhile, the high-resolution TEM (HRTEM) image of the Ni5Pt5- CeO2 (Fig. 4f) clearly shows lattice fringe with d-spacing of about 0.31 nm, which is belong to the (111) plane of CeO2. In addition, the lattice fringe with d-spacing of 0.22 nm is observed (Fig. 4g), which is between the fcc (111) plane of Ni (0.2 nm) and Pt (0.23), further indicating the formation of NiPt alloy. Furthermore, as shown in the Fig. S5 (Supporting information), the energy-dispersive X-ray (EDX) further confirms the co-existence of Ni, Pt, and Ce. Moreover, after durability test, the Ni5Pt5-CeO2 catalyst was also characterized by TEM. As shown in Fig. S6 (Supporting information), the morphology of the Ni5Pt5-CeO2 is maintained well. However, some aggregation is observed, which might be the reason for the slight decrease of activity during the durability test.
|
Download:
|
| Fig. 4. (a, b) SEM images, (c–e) TEM images, (f, g) the magnified HRTEM images in (e). | |
In summary, NiPt NPs have been successfully loading on CeO2 nanospheres through a simple in situ co-reduction method. The obtained Ni5Pt5-CeO2 catalyst exhibits 100% hydrogen selectivity and the highest catalytic activity for dehydrogenation of hydrous hydrazine in an alkaline solution at room temperature, with a TOF value of 416 h-1, which is higher than most of reported catalysts so far. The superior catalytic performance is probably owing to the strong support-metal interaction and synergistic electronic effect between CeO2 and NiPt. This work might open up new avenues for the design of efficient catalysts for dehydrogenation of hydrazine at room temperature, and promote the practical application of hydrazine as promising hydrogen storage material.
AcknowledgmentsThis work was financially supported by the National Natural Science Foundation of China (No. 21571145) and Large-scale Instrument and Equipment Sharing Foundation of Wuhan University.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.11.010.
| [1] |
N. Armaroli, V. Balzani, Angew Chem. Int. Ed. 46 (2007) 52-66. DOI:10.1002/(ISSN)1521-3773 |
| [2] |
S. Peng, G. Jin, L. Li, et al., Chem. Soc. Rev. 45 (2016) 1225-1241. DOI:10.1039/C5CS00777A |
| [3] |
M.P. Suh, H.J. Park, T.K. Prasad, et al., Chem. Rev. 112 (2011) 782-835. |
| [4] |
A. Boddien, D. Mellmann, ärtner F.G., et al., Science 333 (2011) 1733-1736. DOI:10.1126/science.1206613 |
| [5] |
J. Graetz, Chem. Soc. Rev. 38 (2009) 73-82. DOI:10.1039/B718842K |
| [6] |
W. Luo, P.G. Campbell, L.N. Zakharov, et al., J. Am. Chem. Soc. 133 (2011) 19326-19329. DOI:10.1021/ja208834v |
| [7] |
D. Pukazhselvan, V. Kumar, S. Singh, Nano Energy 1 (2012) 566-589. DOI:10.1016/j.nanoen.2012.05.004 |
| [8] |
Y. Du, Y. Shen, Y. Zhan, et al., Chin. Chem. Lett. 28 (2017) 1746-1750. DOI:10.1016/j.cclet.2017.05.018 |
| [9] |
J. Wang, X.B. Zhang, Z.L. Wang, et al., Energy Environ. Sci. 5 (2012) 6885-6888. DOI:10.1039/c2ee03344e |
| [10] |
T. Umegaki, J.M. Yan, X.B. Zhang, et al., Int. J. Hydrogen Energy 34 (2009) 2303-2311. DOI:10.1016/j.ijhydene.2009.01.002 |
| [11] |
L. Wen, Z. Zheng, W. Luo, et al., Chin. Chem. Lett. 26 (2015) 1345-1350. DOI:10.1016/j.cclet.2015.06.019 |
| [12] |
Y. Men, J. Su, C. Huang, et al., Chin. Chem. Lett. 29 (2018) 1671-1674. DOI:10.1016/j.cclet.2018.04.009 |
| [13] |
A. Staubitz, A.P.M. Robertson, I. Manners, Chem. Rev. 110 (2010) 4079-4124. DOI:10.1021/cr100088b |
| [14] |
S.K. Singh, X.B. Zhang, Q. Xu, J. Am. Chem. Soc. 131 (2009) 9894-9895. DOI:10.1021/ja903869y |
| [15] |
B.Q. Xia, K. Chen, W. Luo, et al., Nano Res. 8 (2015) 3472-3479. DOI:10.1007/s12274-015-0845-4 |
| [16] |
Y. Men, X. Du, G. Cheng, et al., Int. J. Hydrogen Energy 42 (2017) 27165-27173. DOI:10.1016/j.ijhydene.2017.08.214 |
| [17] |
S.K. Singh, Q. Xu, Catal. Sci. Technol. 3 (2013) 1889-1900. DOI:10.1039/c3cy00101f |
| [18] |
S.N. Oliaee, C. Zhang, S.Y. Hwang, et al., J. Phys. Chem. C 120 (2016) 9764-9772. DOI:10.1021/acs.jpcc.6b00815 |
| [19] |
J.M. Chen, Z.H. Lu, W. Huang, et al., J. Alloys Compd. 695 (2017) 3036-3043. DOI:10.1016/j.jallcom.2016.11.351 |
| [20] |
Z.J. Zhang, Y.Q. Wang, X.S. Chen, et al., J. Power Sources 291 (2015) 14-19. DOI:10.1016/j.jpowsour.2015.05.012 |
| [21] |
Z.J. Zhang, Z.H. Lu, X.S. Chen, ACS Sustain. Chem. Eng. 3 (2015) 1255-1261. DOI:10.1021/acssuschemeng.5b00250 |
| [22] |
Z. Zhang, S. Zhang, Q. Yao, et al., Inorg. Chem. 56 (2017) 11938-11945. DOI:10.1021/acs.inorgchem.7b01910 |
| [23] |
S.K. Singh, Q. Xu, Inorg. Chem. 49 (2010) 6148-6152. DOI:10.1021/ic1007654 |
| [24] |
H.L. Wang, J.M. Yan, Z.L. Wang, et al., J. Mater. Chem. A 1 (2013) 14957-14962. DOI:10.1039/c3ta13259e |
| [25] |
Y.J. Zhong, H.B. Dai, Y.Y. Jiang, et al., J. Power Sources 300 (2015) 294-300. DOI:10.1016/j.jpowsour.2015.09.071 |
| [26] |
N. Cao, L. Yang, H. Dai, et al., Inorg. Chem. 53 (2014) 10122-10128. DOI:10.1021/ic5010352 |
| [27] |
V.B. Mortola, S. Damyanova, D. Zanchet, et al., Appl. Catal. B:Environ. 107 (2011) 221-236. DOI:10.1016/j.apcatb.2011.07.012 |
| [28] |
W. Tang, Z. Hu, M. Wang, et al., J. Catal. 273 (2010) 125-137. DOI:10.1016/j.jcat.2010.05.005 |
| [29] |
R.K. Singha, S. Ghosh, S.S. Acharyya, et al., Catal. Sci. Technol. 6 (2016) 4601-4615. DOI:10.1039/C5CY02088C |
| [30] |
G. Pantaleo, V. La.Parola, F. Deganello, et al., Appl. Catal. B:Environ. 189 (2016) 233-241. DOI:10.1016/j.apcatb.2016.02.064 |
| [31] |
A. Scarabello, N.D. Dalle, P Canu, et al., Appl. Catal. B:Environ. 174 (2015) 308-322. |
| [32] |
R.K. Singha, A. Shukla, A. Yadav, et al., Catal. Sci. Technol. 7 (2017) 4720-4735. DOI:10.1039/C7CY01493G |
| [33] |
S.K. Singh, A.K. Singh, K. Aranishi, J. Am. Chem. Soc. 133 (2011) 19638-19641. DOI:10.1021/ja208475y |
| [34] |
J. Wang, X.B. Zhang, Z.L. Wang, et al., Energy Environ. Sci. 5 (2012) 6885-6888. DOI:10.1039/c2ee03344e |
| [35] |
C.P. Li, A. Proctor, D.M. Hercules, Appl. Spectrosc. 38 (1984) 880-886. DOI:10.1366/0003702844554530 |
| [36] |
W.J. Wang, M.H. Qiao, H.X. Li, et al., Environ. Clean Technol. 72 (1998) 280-284. |
| [37] |
N. Cao, L. Yang, C. Du, et al., J. Mater. Chem. A 2 (2014) 14344-14347. DOI:10.1039/C4TA02964J |
| [38] |
G.M. Bancroft, I. Adams, L.L. Coatsworth, Anal. Chem. 47 (1975) 586-588. DOI:10.1021/ac60353a050 |
| [39] |
M. Romeo, J. Majerus, P. Légaré, et al., Surf. Sci. 238 (1990) 163-168. DOI:10.1016/0039-6028(90)90073-H |
| [40] |
X. Liu, K. Zhou, L. Wang, et al., J. Am. Chem. Soc. 131 (2009) 3140-3141. DOI:10.1021/ja808433d |
| [41] |
D.D. Sarma, C. Rao, Relat. Phenom. 20 (1980) 25. DOI:10.1016/0368-2048(80)85003-1 |
| [42] |
A. Dauscher, L. Hilaire, N.F. Le, Surf. Interface Anal. 16 (1990) 341-346. DOI:10.1002/(ISSN)1096-9918 |
| [43] |
G. Praline, B.E. Koel, R.L. Hance, et al., J. Electron. Spectrosc. Relat. Phenom. 21 (1980) 17-30. DOI:10.1016/0368-2048(80)85034-1 |
| [44] |
G.M. Ingo, E. Paparazzo, O. Bagnarelli, Surf. Interface Anal. 16 (1990) 515-519. DOI:10.1002/(ISSN)1096-9918 |
| [45] |
G. Praline, B.E. Koel, R.L. Hance, et al., J. Electron. Spectrosc. Relat. Phenom. 21 (1980) 17-30. DOI:10.1016/0368-2048(80)85034-1 |
| [46] |
X.Q. Du, S.Y. Tan, P. Cai, et al., J. Mater. Chem. A 4 (2016) 14572-14576. DOI:10.1039/C6TA05917A |
 2019, Vol. 30
2019, Vol. 30 

