b State Key Laboratory of Bio-Organic and Natural Products Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
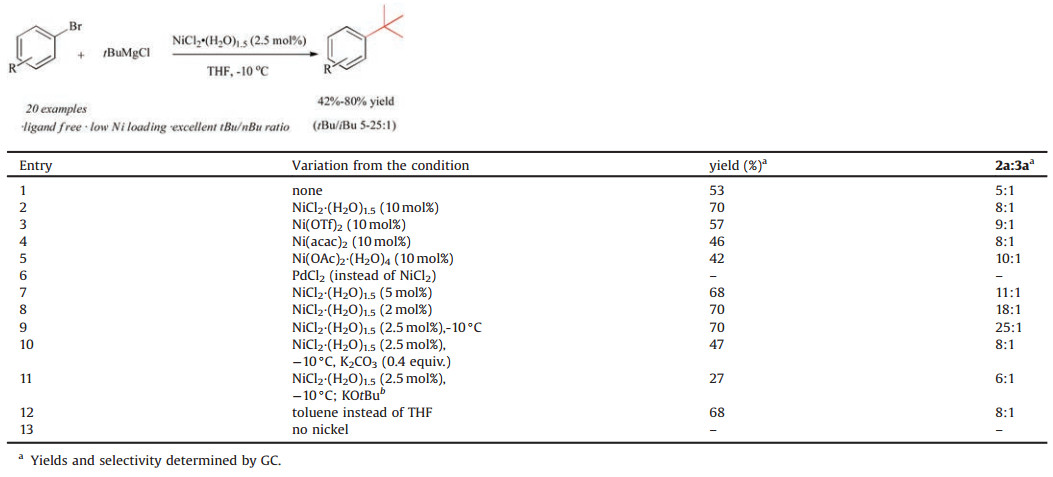
Metal-catalyzed cross-coupling reactions are among the most versatile and important transformations that have found numerous applications in organic synthesis [1]. Although major activities are related to the formation of C(sp2)-C(sp2) bonds, recent advances in transition metal catalysis have enabled the employment of C(sp3) nucleophiles and electrophiles for cross-coupling to form all-carbon quaternary centers [2]. As a part of our ongoing efforts in exploring aryl-alkyl cross-coupling, we have recently reported a Pd-catalyzed Suzuki reaction, allowing the crosscoupling of acyclic secondary alkylboronic acids and aryl bromides to form tertiary carbons in high yields and excellent selectivities [3]. Further research is directed on nickel-catalyzed cross-coupling of tertiary alkyl nucleophiles and aryl halides to form arylsubstituted all-carbon quaternary centers. However, only a few reports of Pd- or Ni-catalyzed cross-couplings employing tertiary alkyl nucleophiles were available and the majority led to the exclusive formation of the isomerization side-products [4] due to rapid β-hydride elimination. Among some notable examples, Kumada and co-workers [5] described a nickel-catalyzed coupling of tertiary alkyl Grignard reagents with β-bromostyrene. Biscoe [6] and Glorius [7] reported respectively the Ni-catalyzed Kumada cross-couplings of tertiary alkyl magnesium halides and aryl bromides by using N-heterocyclic carbenes (NHC) as supporting ligands (Scheme 1). Nevertheless, high loadings of catalysts and ligands are necessary in their reports, which hampered the practicality and applications of such methods. We herein report that the cross-coupling proceed under ligand-free conditions even at a lower loading of nickel. Herein, we detailed our results on efficient Ni-catalyzed cross-couplings of tertiary alkyl Grignard reagents with aryl bromides.
|
Download:
|
| Scheme 1. Kumada coupling of tertiary alkyl Grignard reagents. | |
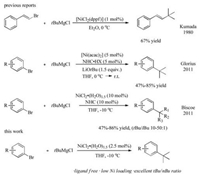
We looked into the Ni-catalyzed cross-coupling of tBuMgCl and 2-bromonaphthalene (1a). The yields and proportions of the potential products (2a-3a) were readily analyzable by gas chromatography. The reactions were conducted at 0 ℃ for 30 min in THF with 10 mol% NiCl2 in the absence of a phosphorus or NHC ligand. We were pleased that the desired cross-coupling product 2a was obtained in 53% yield with a 2a/3a ratio of 5:1. Various nickel precursors were investigated and NiCl2·(H2O)1.5 proved to be best in terms of yield (Table 1, entries 2–5). Employment of PdCl2 instead of NiCl2 gave no desired product (entry 6). We thus selected NiCl2·(H2O)1.5 as the nickel precursor for further optimization. When the Ni loading decreased to 2 mol%, we found that a good yield maintained while the 2a/3a selectivity was further improved (entries 7 and 8). By reducing the reaction temperature to -10 ℃ and increasing the Ni loading to 2.5 mol%, the cross-coupling product 2a was afforded in 70% yield with a 2a/3a ratio of 25:1 (entry 9). Further addition of K2CO3 or KOtBu did not result in an improved yield (entries 10 and 11). No formation of the product was observed in the absence of a nickel catalyst.
|
|
Table 1 Optimization of the Ni-catalyzed cross-coupling of tBuMgCl and 2-bromonaphthlene. |
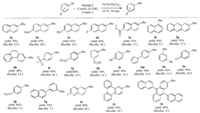
The substrate scope of the Ni-catalyzed coupling of aryl bromides with tertiary alkyl magnesium halides was explored. As can be seen in Scheme 2, both electron-rich (2b and 2c) and electron-deficient (2d and 2e) naphthalene products were isolated in moderate to good yields and excellent tBu/iBu ratios (10-25:1). Functional groups such as ester (2e, 2j, and 2k), pyrrole (2m), sulfonyl group (2i), and trifluoromethoxy group (2p) were well tolerable under the reaction conditions. The method also displayed good chemo-selectivity on aryl bromide over aryl chloride or fluoride as indicated by selective preparation of products 2d and 2o in decent yields. Thus, chloro- and fluoro-aryl substituted hydrocarbons bearing all-carbon quaternary centers could be easily formed, which were viable for further derivatization. We were delighted that the coupling could also occur on 1-bromonaphthalene, leading to the formation of 2f in a moderate yield, indicating the tolerance of certain steric hindrance of the reaction on substrate. The coupling was also compatible with large aromatic systems such as anthracene (2g and 2t), phenanthrene (2h), and triphenylene (2s). para- and meta-Substituted bromo benzenes also gave coupling products in moderate to excellent yields and good tBu/iBu ratios (2i-2q). No coupling product was observed with ortho-methyl bromo benzene as the substrate, possible due to steric hindrance. When a heteroaryl bromide was employed in this reaction, we were pleased that a quinoline product (2r) was formed in a satisfactory yield (80%). A pyridine derivative 4- bromo-2, 6-lutidine could also be employed to provide the coupling product in 50% yield with a tBu/iBu ratio of 4:1.
|
Download:
|
| Scheme 2. Ni-catalyzed cross-coupling of tBuMgCl and Aryl bromides. Unless otherwise stated, the reactions were performed in THF under 4 equiv. tBuMgCl at -10 ℃ for 30 min in the presence of 2.5 mol% NiCl2·(H2O)1.5; isolated yield was given; ratio determined by 1H NMR spectroscopy. | |
To demonstrate the practicality of this method, the coupling was performed at a gram scale. In the presence of 2.5 mol% NiCl2·(H2O)1.5, the reaction was complete in 30 min and the coupling product 2a was obtained in 62% isolated yield with a tBu/iBu ratio of 15:1 (Scheme 3).

|
Download:
|
| Scheme 3. Gram-scale of Ni-catalyzed cross-coupling. | |
To shed light on the mechanism of this transformation, the following experiments were implemented. Under similar reaction conditions with additional addition of TEMPO or DMPO (2 equiv.), little or no coupling product was obtained (Scheme 4). When para- methyl bromobenzene (1u) was employed as the substrate, a significant amount of the homo-coupling product 2u' was formed in 40% yield. When 1-bromo-2-(but-3-en-1-yl)benzene (1v) was employed as the substrate, a cyclization product 2v was formed. These experiments indicated a possible radical process during the coupling.

|
Download:
|
| Scheme 4. Mechanistic investigation. | |
On the basis of Louie's proposal [8] as well as our own investigations, we proposed a mechanism of the ligand-free nickelcatalyzed cross-coupling of aryl bromides with tert-butyl Grignard reagent illustrated in Scheme 5. Reduction of Ni(Ⅱ) species by Grignard reagent forms a tBu-Ni(Ⅰ) species A. Oxidative addition of aryl bromide to A affords a Ni(Ⅲ) species B, which undergoes a normal reductive elimination pathway to form the cross-coupling product tBu-Ar and Ni(Ⅰ)-X species E. Transmetallation of E with tert-butyl Grignard reagent regenerates tBu-Ni(Ⅰ) species and completes the catalytic cycle. When an electron-rich aryl bromide such as 1u is employed, the normal pathway of reductive elimination slows down and the undesired pathway of reductive elimination to eliminate tBuBr takes place to form Ar-Ni(Ⅰ) (C), which could further react with ArBr to form Ni(Ⅲ) species D and lead to the formation of homocoupling side-product Ar-Ar by reductive elimination.

|
Download:
|
| Scheme 5. Proposed mechanism. | |
In summary, we have developed a ligand-free Ni-catalyzed coupling of tert-butyl Grignard reagent and aryl bromides that have shown moderate to good yield with good to excellent tBu/iBu ratios. This process allows facile and efficient construction of arylsubstituted hydrocarbons with all-carbon quaternary centers at a low nickel loading. A radical coupling process is indicated and a mechanism with a Ni(Ⅰ)-Ni(Ⅲ) catalytic cycle is proposed. Further mechanistic investigation is ongoing in our laboratory.
AcknowledgmentsWe are grateful to the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB20000000), CAS (No. QYZDY-SSW-SLH029), National Natural Science Foundation of China (Nos. 21725205, 21432007, 21572246), STCSM- 18520712200, and K.C. Wong Education Foundation.
Appendix A. Supplementary dataSupplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.cclet.2018.12.027.
| [1] |
(a) A. de Meijere, F. Diederich, Metal-Catalyzed Cross-Coupling Reactions, Wiley-VCH, New York, 2004; (b) M. Beller, C. Bolm (Eds.), Transition Metals for Organic Synthesis, Wiley-VCH, Weinheim, 2004; (c) A. Rudolph, M. Lautens, Angew. Chem. Int. Ed. 48 (2009) 2656-2670. |
| [2] |
(a) T.W. Bell, L. Hu, S.V. Patelf, J. Org. Chem. 52 (1987) 3847-3850; (b) S.R. Chemler, D. Trauner, S.J. Danishefsky, Angew. Chem. Int. Ed. 40 (2001) 4544-4568; (c) M.R. Netherton, G.C. Fu, in: J. Tsuji (Ed.), Topics in Organometallic Chemistry: Palladium in Organic Synthesis, Springer, New York, 2005, pp. 85-108; (d) S.L. Zultanski, G.C. Fu, J. Am. Chem. Soc. 135 (2013) 624-627; (e) X. Wang, S. Wang, W. Xue, H. Gong, J. Am. Chem. Soc. 137 (2015) 11562-11565; (f) S. Ando, M. Mawatari, H. Matsunaga, T. Ishizuka, Tetrahedron Lett. 57 (2016) 3287-3290; (g) G. Wang, R. Shang, W. Cheng, Y. Fu, J. Am. Chem. Soc. 139 (2017) 18307-18312; (h) D.N. Primer, G.A. Molander, J. Am. Chem. Soc. 139 (2017) 9847-9850; (i) Z.T. Ariki, Y. Maekawa, M. Nambo, C.M. Crudden, J. Am. Chem. Soc. 140 (2018) 78-81. |
| [3] |
(a) C. Li, T. Chen, W. Tang, Angew. Chem. Int. Ed. 54 (2015) 3792-3796; (b) T. Si, B. Li, W. Xiong, B. Xu, W. Tang, Org. Biomol. Chem.15 (2017) 9903-9909. |
| [4] |
(a) X. Luo, H. Zhang, H. Duan, et al., Org. Lett. 9 (2007) 4571-4574; (b) J. Breitenfeld, O. Vechorkin, C. Corminboeuf, R. Scopelliti, X. Hu, Organometallics 29 (2010) 3686-3689. |
| [5] |
T. Hayashi, M. Konishi, K. Yokota, M. Kumada, Chem. Lett. 9 (1980) 767-768. DOI:10.1246/cl.1980.767 |
| [6] |
A. Joshi-Pangu, C. Wang, M.R. Biscoe, J. Am. Chem. Soc. 133 (2011) 8478-8481. DOI:10.1021/ja202769t |
| [7] |
C. Lohre, T. Dröge, C. Wang, F. Glorius, Chem.-Eur. J. 17 (2011) 6052-6055. DOI:10.1002/chem.v17.22 |
| [8] |
(a) T.J. Anderson, G.D. Jones, D.A. Vicic, J. Am. Chem. Soc. 126 (2004) 8100-8101; (b) G.D. Jones, J.L. Martin, C. McFarland, et al., J. Am. Chem. Soc. 128 (2006) 13175-13183; (c) V.B. Phapale, M. Guisán-Ceinos, E. Buñuel, D.J. Cárdenas, Chem.-Eur. J. 15 (2009) 12681-12688; (d) K. Zhang, M.C. Sheridan, S.R. Cooke, J. Louie, Organometallics 30 (2011) 2546-2552; (e) F. Han, Chem. Soc. Rev. 42 (2013) 5270-5298; (f) S. Biswas, D.J. Weix, J. Am. Chem. Soc. 135 (2013) 16192-16197; (g) K.C. Shekhar, R.K. Dhungana, B. Shrestha, et al., J. Am. Chem. Soc. 140 (2018) 9801-9805. |
 2019, Vol. 30
2019, Vol. 30